Boxed Warning
WARNING: HEPATOTOXICITY and INTRACRANIAL HEMORRHAGE
Hepatotoxicity:
Clinical hepatitis and hepatic decompensation, including some fatalities, have been reported. Extra vigilance is warranted in patients with chronic hepatitis B or hepatitis C co-infection, as these patients have an increased risk of hepatotoxicity [see Warnings and Precautions (5.1) ].
Intracranial Hemorrhage:
Both fatal and non-fatal intracranial hemorrhage have been reported [see Warnings and Precautions (5.2) ].
1. Indications and Usage
APTIVUS, co-administered with ritonavir, is indicated for combination antiretroviral treatment of HIV-1 infected patients who are treatment-experienced and infected with HIV-1 strains resistant to more than one protease inhibitor (PI).
This indication is based on analyses of plasma HIV-1 RNA levels in two controlled studies of APTIVUS/ritonavir of 48 weeks duration in treatment-experienced adults and one open-label 48-week study in pediatric patients age 2 to 18 years. The adult studies were conducted in clinically advanced, 3-class antiretroviral (NRTI, NNRTI, PI) treatment-experienced adults with evidence of HIV-1 replication despite ongoing antiretroviral therapy.
The following points should be considered when initiating therapy with APTIVUS/ritonavir:
- The use of APTIVUS/ritonavir in treatment-naïve patients is not recommended [see Warnings and Precautions (5.1) ].
- The use of other active agents with APTIVUS/ritonavir is associated with a greater likelihood of treatment response [see Microbiology (12.4) and Clinical Studies (14) ].
- Genotypic or phenotypic testing and/or treatment history should guide the use of APTIVUS/ritonavir [see Microbiology (12.4) ]. The number of baseline primary protease inhibitor mutations affects the virologic response to APTIVUS/ritonavir [see Microbiology (12.4) ].
- Use caution when prescribing APTIVUS/ritonavir to patients with elevated transaminases, hepatitis B or C co-infection or patients with mild hepatic impairment [see Warnings and Precautions (5.1) ].
- Liver function tests should be performed at initiation of therapy with APTIVUS/ritonavir and monitored frequently throughout the duration of treatment [see Warnings and Precautions (5.1) ].
- The drug-drug interaction potential of APTIVUS/ritonavir when co-administered with other drugs must be considered prior to and during APTIVUS/ritonavir use [see Contraindications (4) and Drug Interactions (7) ].
- Use caution when prescribing APTIVUS/ritonavir in patients who may be at risk for increased bleeding or who are receiving medications known to increase the risk of bleeding [see Warnings and Precautions (5.4) ].
- The risk-benefit of APTIVUS/ritonavir has not been established in pediatric patients <2 years of age.
There are no study results demonstrating the effect of APTIVUS/ritonavir on clinical progression of HIV-1.
2. Dosage and Administration
APTIVUS must be co-administered with ritonavir to exert its therapeutic effect. Failure to correctly co-administer APTIVUS with ritonavir will result in plasma levels of tipranavir that will be insufficient to achieve the desired antiviral effect and will alter some drug interactions.
- APTIVUS co-administered with ritonavir capsules or solution can be taken with or without meals
- APTIVUS co-administered with ritonavir tablets must only be taken with meals
[see Clinical Pharmacology (12.3) ]
APTIVUS may be administered as either capsules or oral solution to either pediatric or adult patients. APTIVUS capsules must be swallowed whole and must not be opened or chewed.
Due to the need for co-administration of APTIVUS with ritonavir, please refer to the ritonavir prescribing information.
2.1 Adults
The recommended adult dose of APTIVUS is 500 mg (two 250 mg capsules or 5 mL oral solution) co-administered with 200 mg of ritonavir, twice daily.
2.2 Pediatric Patients (age 2 to 18 years)
Healthcare professionals should pay special attention to accurate calculation of the dose of APTIVUS, transcription of the medication order, dispensing information and dosing instruction to minimize risk for medication errors, overdose, and underdose.
Prescribers should calculate the appropriate dose of APTIVUS for each individual child based on body weight (kg) or body surface area (BSA, m2) and should not exceed the recommended adult dose.
Before prescribing APTIVUS 250 mg capsules, children should be assessed for the ability to swallow capsules. If a child is unable to reliably swallow an APTIVUS capsule, the APTIVUS oral solution formulation should be prescribed.
The recommended pediatric dose of APTIVUS is 14 mg/kg with 6 mg/kg ritonavir (or 375 mg/m2 co-administered with ritonavir 150 mg/m2) taken twice daily not to exceed a maximum dose of APTIVUS 500 mg co-administered with ritonavir 200 mg twice daily. For children who develop intolerance or toxicity and cannot continue with APTIVUS 14 mg/kg with 6 mg/kg ritonavir, physicians may consider decreasing the dose to APTIVUS 12 mg/kg with 5 mg/kg ritonavir (or APTIVUS 290 mg/m2 co-administered with 115 mg/m2 ritonavir) taken twice daily provided their virus is not resistant to multiple protease inhibitors [see Adverse Reactions (6.2), Use in Specific Populations (8.4) , and Clinical Studies (14.2) ].
Body surface area can be calculated as follows:
| Mosteller Formula: | BSA (m2) = | 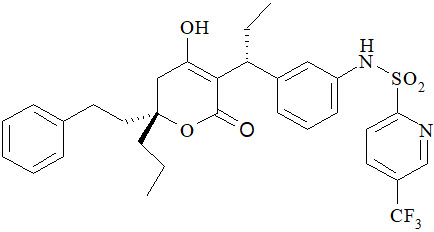 |
3. Dosage Forms and Strengths
4. Contraindications
- APTIVUS is contraindicated in patients with moderate or severe (Child-Pugh Class B or C, respectively) hepatic impairment [see Warnings and Precautions (5.1) ].
- APTIVUS/ritonavir is contraindicated when co-administered with drugs that are highly dependent on CYP 3A for clearance or are potent CYP 3A inducers (see Table 1) [see Drug Interactions (7.2) ].
| Drug Class | Drugs within Class that are Contraindicated with APTIVUS Co-administered with Ritonavir | Clinical Comments: |
|---|---|---|
| Alpha 1-adrenoreceptor antagonist | Alfuzosin | Potentially increased alfuzosin concentrations can result in hypotension. |
| Antiarrhythmics | Amiodarone, bepridil, flecainide, propafenone, quinidine | Potential for serious and/or life-threatening reactions such as cardiac arrhythmias secondary to increases in plasma concentrations of antiarrhythmics. |
| Antimycobacterials | Rifampin | May lead to loss of virologic response and possible resistance to APTIVUS or to the class of protease inhibitors or other co-administered antiretroviral agents. |
| Ergot derivatives | Dihydroergotamine, ergonovine, ergotamine, methylergonovine | Potential for acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
| GI motility agent | Cisapride | Potential for cardiac arrhythmias. |
| Herbal products | St. John's wort (hypericum perforatum) | May lead to loss of virologic response and possible resistance to APTIVUS or to the class of protease inhibitors. |
| HMG CoA reductase inhibitors | Lovastatin, simvastatin | Potential for myopathy including rhabdomyolysis. |
| Antipsychotics | Pimozide | Potential for cardiac arrhythmias. |
| Lurasidone | Potential for serious and/or life-threatening reactions. | |
| PDE-5 inhibitors | Sildenafil (Revatio) [for treatment of pulmonary arterial hypertension] | A safe and effective dose has not been established when used with APTIVUS/ritonavir. There is increased potential for sildenafil-associated adverse events (which include visual disturbances, hypotension, prolonged erection, and syncope). |
| Sedatives/hypnotics | Oral midazolam, triazolam | Prolonged or increased sedation or respiratory depression. |
Due to the need for co-administration of APTIVUS with ritonavir, please refer to the ritonavir prescribing information for a description of ritonavir contraindications.
5. Warnings and Precautions
Please refer to the ritonavir prescribing information for additional information on precautionary measures.
5.1 Hepatic Impairment and Toxicity
Clinical hepatitis and hepatic decompensation, including some fatalities, were reported with APTIVUS co-administered with 200 mg of ritonavir. These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications. A causal relationship to APTIVUS/ritonavir could not be established. Physicians and patients should be vigilant for the appearance of signs or symptoms of hepatitis, such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. Patients with signs or symptoms of clinical hepatitis should discontinue APTIVUS/ritonavir treatment and seek medical evaluation.
All patients should be followed closely with clinical and laboratory monitoring, especially those with chronic hepatitis B or C co-infection, as these patients have an increased risk of hepatotoxicity. Liver function tests should be performed prior to initiating therapy with APTIVUS/ritonavir, and frequently throughout the duration of treatment.
If asymptomatic elevations in AST or ALT greater than 10 times the upper limit of normal occur, APTIVUS/ritonavir therapy should be discontinued. If asymptomatic elevations in AST or ALT between 5 – 10 times the upper limit of normal and increases in total bilirubin greater than 2.5 times the upper limit of normal occur, APTIVUS/ritonavir therapy should be discontinued.
Treatment-experienced patients with chronic hepatitis B or hepatitis C co-infection or elevated transaminases are at approximately 2-fold risk for developing Grade 3 or 4 transaminase elevations or hepatic decompensation. In two large, randomized, open-label, controlled clinical trials with an active comparator (1182.12 and 1182.48) of treatment-experienced patients, Grade 3 and 4 increases in hepatic transaminases were observed in 10.3% (10.9/100 PEY) receiving APTIVUS/ritonavir through week 48. In a study of treatment-naïve patients, 20.3% (21/100 PEY) experienced Grade 3 or 4 hepatic transaminase elevations while receiving APTIVUS/ritonavir 500 mg/200 mg through week 48.
Tipranavir is principally metabolized by the liver. Caution should be exercised when administering APTIVUS/ritonavir to patients with mild hepatic impairment (Child-Pugh Class A) because tipranavir concentrations may be increased [see Clinical Pharmacology (12.3) ].
5.2 Intracranial Hemorrhage
APTIVUS, co-administered with 200 mg of ritonavir, has been associated with reports of both fatal and non-fatal intracranial hemorrhage (ICH). Many of these patients had other medical conditions or were receiving concomitant medications that may have caused or contributed to these events. No pattern of abnormal coagulation parameters has been observed in patients in general, or preceding the development of ICH. Therefore, routine measurement of coagulation parameters is not currently indicated in the management of patients on APTIVUS.
5.3 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of APTIVUS/ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving APTIVUS/ritonavir, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of APTIVUS/ritonavir, respectively. These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of APTIVUS/ritonavir.
- Loss of therapeutic effect of APTIVUS/ritonavir and possible development of resistance.
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7) ]. Consider the potential for drug interactions prior to and during APTIVUS/ritonavir therapy; review concomitant medications during APTIVUS/ritonavir therapy; and monitor for the adverse reactions associated with the concomitant medications [see Contraindications (4) and Drug Interactions (7) ].
5.4 Effects on Platelet Aggregation and Coagulation
APTIVUS/ritonavir should be used with caution in patients who may be at risk of increased bleeding from trauma, surgery or other medical conditions, or who are receiving medications known to increase the risk of bleeding such as antiplatelet agents and anticoagulants, or who are taking supplemental high doses of vitamin E.
In rats, tipranavir treatment alone induced dose-dependent changes in coagulation parameters, bleeding events and death. Co-administration with vitamin E significantly increased these effects [see Nonclinical Toxicology (13.2) ]. However, analyses of stored plasma from adult patients treated with APTIVUS capsules and pediatric patients treated with APTIVUS oral solution (which contains a vitamin E derivative) showed no effect of APTIVUS/ritonavir on vitamin K-dependent coagulation factors (Factor II and Factor VII), Factor V, or on prothrombin or activated partial thromboplastin times.
In in vitro experiments, tipranavir was observed to inhibit human platelet aggregation at levels consistent with exposures observed in patients receiving APTIVUS/ritonavir.
5.5 Vitamin E Intake
Patients taking APTIVUS oral solution should be advised not to take supplemental vitamin E greater than a standard multivitamin as APTIVUS oral solution contains 116 IU/mL of vitamin E which is higher than the Reference Daily Intake (adults 30 IU, pediatrics approximately 10 IU).
5.6 Rash
Rash, including urticarial rash, maculopapular rash, and possible photosensitivity, has been reported in subjects receiving APTIVUS/ritonavir. In some cases rash was accompanied by joint pain or stiffness, throat tightness, or generalized pruritus. In controlled adult clinical trials, rash (all grades, all causality) was observed in 10% of females and in 8% of males receiving APTIVUS/ritonavir through 48 weeks of treatment. The median time to onset of rash was 53 days and the median duration of rash was 22 days. The discontinuation rate for rash in clinical trials was 0.5%. In an uncontrolled compassionate use program (n=3920), cases of rash, some of which were severe, accompanied by myalgia, fever, erythema, desquamation, and mucosal erosions were reported. In the pediatric clinical trial, the frequency of rash (all grades, all causality) through 48 weeks of treatment was 21%. Overall, most of the pediatric patients had mild rash and 5 (5%) had moderate rash. Overall 3% of pediatric patients interrupted APTIVUS treatment due to rash and the discontinuation rate for rash in pediatric patients was 0.9%. Discontinue and initiate appropriate treatment if severe skin rash develops.
5.7 Sulfa Allergy
APTIVUS should be used with caution in patients with a known sulfonamide allergy. Tipranavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and APTIVUS is unknown.
5.8 Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-1 infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
5.9 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including APTIVUS. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia, tuberculosis, or reactivation of herpes simplex and herpes zoster), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.10 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.11 Elevated Lipids
Treatment with APTIVUS co-administered with 200 mg of ritonavir has resulted in large increases in the concentration of total cholesterol and triglycerides [see Adverse Reactions (6) ]. Triglyceride and cholesterol testing should be performed prior to initiating APTIVUS/ritonavir therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate; taking into account any potential drug-drug interactions [see Drug Interactions (7.2) ].
5.12 Patients with Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional Factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced if treatment had been discontinued. A causal relationship between protease inhibitors and these events has not been established.
6. Adverse Reactions
The following adverse reactions are described, in greater detail, in other sections:
- Hepatic Impairment and Toxicity [see Warnings and Precautions (5.1) ]
- Intracranial Hemorrhage [see Warnings and Precautions (5.2) ]
- Rash [see Warnings and Precautions (5.6) ]
Due to the need for co-administration of APTIVUS with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Trials in Adults
APTIVUS, co-administered with ritonavir, has been studied in a total of 6308 HIV-1 positive adults as combination therapy in clinical studies. Of these, 1299 treatment-experienced patients received the dose of 500/200 mg BID. Nine hundred nine (909) adults, including 541 in the 1182.12 and 1182.48 controlled clinical trials, have been treated for at least 48 weeks [see Clinical Studies (14) ].
In 1182.12 and 1182.48 in the APTIVUS/ritonavir arm, the most frequent adverse reactions were diarrhea, nausea, pyrexia, vomiting, fatigue, headache, and abdominal pain. The 48-Week Kaplan-Meier rates of adverse reactions leading to discontinuation were 13.3% for APTIVUS/ritonavir-treated patients and 10.8% for the comparator arm patients.
Adverse reactions reported in the controlled clinical trials 1182.12 and 1182.48, based on treatment-emergent clinical adverse reactions of moderate to severe intensity (Grades 2 - 4) in at least 2% of treatment-experienced subjects in either treatment group are summarized in Table 2 below.
| Percentage of patients (rate per 100 patient-exposure years) | ||
|---|---|---|
| APTIVUS/ritonavir (500/200 mg BID) + OBRc
(n=749; 757.4 patient-exposure years) | Comparator PI/ritonavirb + OBR (n=737; 503.9 patient-exposure years) | |
| aExcludes laboratory abnormalities that were Adverse Events | ||
| bComparator PI/ritonavir: lopinavir/ritonavir 400/100 mg BID, indinavir/ritonavir 800/100 mg BID, saquinavir/ritonavir 1000/100 mg BID, amprenavir/ritonavir 600/100 mg BID | ||
| cOptimized Background Regimen | ||
| Blood and Lymphatic Disorders | ||
| Anemia | 3.3% (3.4) | 2.3% (3.4) |
| Neutropenia | 2.0% (2.0) | 1.0% (1.4) |
| Gastrointestinal Disorders | ||
| Diarrhea | 15.0% (16.5) | 13.4% (21.6) |
| Nausea | 8.5% (9.0) | 6.4% (9.7) |
| Vomiting | 5.9% (6.0) | 4.1% (6.1) |
| Abdominal pain | 4.4% (4.5) | 3.4% (5.1) |
| Abdominal pain upper | 1.5% (1.5) | 2.3% (3.4) |
| General Disorders | ||
| Pyrexia | 7.5% (7.7) | 5.4% (8.2) |
| Fatigue | 5.7% (5.9) | 5.6% (8.4) |
| Investigations | ||
| Weight decreased | 3.1% (3.1) | 2.2% (3.2) |
| ALT increased | 2.0% (2.0) | 0.5% (0.8) |
| GGT increased | 2.0% (2.0) | 0.4% (0.6) |
| Metabolism and Nutrition Disorders | ||
| Hypertriglyceridemia | 3.9% (4.0) | 2.0% (3.0) |
| Hyperlipidemia | 2.5% (2.6) | 0.8% (1.2) |
| Dehydration | 2.1% (2.1) | 1.1% (1.6) |
| Musculoskeletal and Connective Tissue Disorders | ||
| Myalgia | 2.3% (2.3) | 1.8% (2.6) |
| Nervous System Disorders | ||
| Headache | 5.2% (5.3) | 4.2% (6.3) |
| Peripheral neuropathy | 1.5% (1.5) | 2.0% (3.0) |
| Psychiatric Disorders | ||
| Insomnia | 1.7% (1.7) | 3.7% (5.5) |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Dyspnea | 2.1% (2.1) | 1.0% (1.4) |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 3.1% (3.1) | 3.8% (5.7) |
Less Common Adverse Reactions
Other adverse reactions reported in <2% of adult patients (n=1474) treated with APTIVUS/ritonavir 500/200 mg in Phase 2 and 3 clinical trials are listed below by body system:
Blood and Lymphatic System Disorders: thrombocytopenia
Gastrointestinal Disorders: abdominal distension, dyspepsia, flatulence, gastroesophageal reflux disease, pancreatitis
General Disorders: influenza-like illness, malaise
Hepatobiliary Disorders: hepatitis, hepatic failure, hyperbilirubinemia, cytolytic hepatitis, toxic hepatitis, hepatic steatosis
Immune System Disorders: hypersensitivity
Investigations: hepatic enzymes increased, liver function test abnormal, lipase increased
Metabolism and Nutrition Disorders: anorexia, decreased appetite, diabetes mellitus, facial wasting, hyperamylasemia, hypercholesterolemia, hyperglycemia, mitochondrial toxicity
Musculoskeletal and Connective Tissue Disorders: muscle cramp
Nervous System Disorders: dizziness, intracranial hemorrhage, somnolence
Psychiatric Disorders: sleep disorder
Renal and Urinary Disorders: renal insufficiency
Skin and Subcutaneous System Disorders: exanthem, lipoatrophy, lipodystrophy acquired, lipohypertrophy, pruritus
Laboratory Abnormalities
Treatment-emergent laboratory abnormalities reported at 48 weeks in the controlled clinical trials 1182.12 and 1182.48 in adults are summarized in Table 3 below.
| Randomized, Controlled Clinical Trials 1182.12 and 1182.48 | |||
|---|---|---|---|
| Percentage of Patients (rate per 100 patient-exposure years) | |||
| Limit | APTIVUS/ritonavir (500/200 mg BID) + OBR (n=738) | Comparator PI/ritonavir + OBR* (n=724) | |
| *Comparator PI/ritonavir: lopinavir/ritonavir 400/100 mg BID, indinavir/ritonavir 800/100 mg BID, saquinavir/ritonavir 1000/100 mg BID, amprenavir/ritonavir 600/100 mg BID | |||
| Hematology | |||
| WBC count decrease | |||
| Grade 3 | <2.0 × 103/µL | 5.4% (5.6) | 4.8% (7.7) |
| Grade 4 | <1.0 × 103/µL | 0.3% (0.3) | 1.1% (1.7) |
| Chemistry | |||
| Amylase | |||
| Grade 3 | >2.5 × ULN | 5.7% (5.9) | 6.4% (10.4) |
| Grade 4 | >5 × ULN | 0.3% (0.3) | 0.7% (1.1) |
| ALT | |||
| Grade 2 | >2.5-5 × ULN | 14.9% (16.5) | 7.5% (12.4) |
| Grade 3 | >5-10 × ULN | 5.6% (5.7) | 1.7% (2.6) |
| Grade 4 | >10 × ULN | 4.1% (4.1) | 0.4% (0.7) |
| AST | |||
| Grade 2 | >2.5-5 × ULN | 9.9% (10.5) | 8.0% (13.3) |
| Grade 3 | >5-10 × ULN | 4.5% (4.6) | 1.4% (2.2) |
| Grade 4 | >10 × ULN | 1.6% (1.6) | 0.4% (0.6) |
| ALT and/or AST | |||
| Grade 2-4 | >2.5 × ULN | 26.0% (31.5) | 13.7% (23.8) |
| Cholesterol | |||
| Grade 2 | >300 – 400 mg/dL | 15.6% (17.7) | 6.4% (10.5) |
| Grade 3 | >400 – 500 mg/dL | 3.3% (3.3) | 0.3% (0.4) |
| Grade 4 | >500 mg/dL | 0.9% (1.0) | 0.1% (0.2) |
| Triglycerides | |||
| Grade 2 | 400 – 750 mg/dL | 35.9% (49.9) | 26.8% (51.0) |
| Grade 3 | >750 – 1200 mg/dL | 16.9% (19.4) | 8.7% (14.6) |
| Grade 4 | >1200 mg/dL | 8.0% (8.4) | 4.3% (7.0) |
In controlled clinical trials 1182.12 and 1182.48 extending up to 96 weeks, the proportion of patients who developed Grade 2-4 ALT and/or AST elevations increased from 26% at week 48 to 32.1% at week 96 with APTIVUS/ritonavir. The risk of developing transaminase elevations is greater during the first year of therapy.
6.2 Clinical Trials in Pediatric Patients
APTIVUS, co-administered with ritonavir, has been studied in a total of 135 HIV-1 infected pediatric patients age 2 through 18 years as combination therapy. This study enrolled HIV-1 infected, treatment-experienced pediatric patients (with the exception of 3 treatment-naïve patients), with baseline HIV-1 RNA of at least 1500 copies/mL. One hundred and ten (110) patients were enrolled in a randomized, open-label 48-week clinical trial (Study 1182.14) and 25 patients were enrolled in other clinical studies including Expanded Access and Emergency Use Programs.
The adverse reactions profile seen in Study 1182.14 was similar to adults. Pyrexia (6.4%), vomiting (5.5%), cough (5.5%), rash (5.5%), nausea (4.5%), and diarrhea (3.6%) were the most frequently reported adverse reactions (Grade 2-4, all causes) in pediatric patients. Rash was reported more frequently in pediatric patients than in adults.
The most common Grade 3-4 laboratory abnormalities were increases in CPK (11%), ALT (6.5%), and amylase (7.5%).
Due to previous reports of both fatal and non-fatal intracranial hemorrhage (ICH), an analysis of bleeding events was performed. At 48 weeks of treatment, the frequency of pediatric patients with any bleeding adverse reactions was 7.5%. No drug related serious bleeding adverse reaction was reported. The most frequent bleeding adverse reaction was epistaxis (3.7%). No other bleeding adverse reaction was reported in frequency of >1%. Additional trial follow-up through 100 weeks showed a cumulative 12% frequency of any bleeding adverse reaction.
7. Drug Interactions
7.1 Potential for APTIVUS/ritonavir to Affect Other Drugs
APTIVUS co-administered with ritonavir at the recommended dose is a net inhibitor of CYP 3A and may increase plasma concentrations of agents that are primarily metabolized by CYP 3A. Thus, co-administration of APTIVUS/ritonavir with drugs highly dependent on CYP 3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated [see Contraindications (4) ]. Co-administration with other CYP 3A substrates may require a dose adjustment or additional monitoring [see Drug Interactions (7) ].
Clinically significant drug-drug interactions of APTIVUS co-administered with ritonavir are summarized in Table 4 below.
A phenotypic cocktail study was conducted with 16 healthy volunteers to quantify the influence of 10 days of APTIVUS/ritonavir capsule administration on the activity of hepatic CYP 1A2 (caffeine), 2C9 (warfarin), 2C19 (omeprazole), 2D6 (dextromethorphan) and the activity of intestinal and hepatic CYP 3A4/5 (midazolam) and P-glycoprotein (P-gp) (digoxin). This study determined the first-dose and steady-state effects of 500 mg of APTIVUS co-administered with 200 mg of ritonavir twice daily in capsule form. APTIVUS oral solution co-administered with ritonavir capsules demonstrated similar effects as APTIVUS capsules co-administrated with ritonavir.
There was no net effect on CYP 2C9 or hepatic P-gp at first dose or steady state. There was no net effect after first dose on CYP 1A2, but there was moderate induction at steady state. There was modest inhibition of CYP 2C19 at the first dose, but there was marked induction at steady state. Potent inhibition of CYP 2D6 and both hepatic and intestinal CYP 3A4/5 activities were observed after first dose and steady state.
Intestinal and hepatic P-gp activity was assessed by administering oral and intravenous digoxin, respectively. The digoxin results indicate P-gp was inhibited after the first dose of APTIVUS/ritonavir followed by induction of P-gp over time. Thus, it is difficult to predict the net effect of APTIVUS administered with ritonavir on oral bioavailability and plasma concentrations of drugs that are dual substrates of CYP 3A and P-gp. The net effect will vary depending on the relative affinity of the co-administered drugs for CYP 3A and P-gp, and the extent of intestinal first-pass metabolism/efflux. An in vitro induction study in human hepatocytes showed an increase in UGT1A1 by tipranavir similar to that evoked by rifampin. The clinical consequences of this finding have not been established.
7.2 Potential for Other Drugs to Affect Tipranavir
Tipranavir is a CYP 3A substrate and a P-gp substrate. Co-administration of APTIVUS/ritonavir and drugs that induce CYP 3A and/or P-gp may decrease tipranavir plasma concentrations. Co-administration of APTIVUS/ritonavir and drugs that inhibit P-gp may increase tipranavir plasma concentrations. Co-administration of APTIVUS/ritonavir with drugs that inhibit CYP 3A may not further increase tipranavir plasma concentrations, because the level of metabolites is low following steady-state administration of APTIVUS/ritonavir 500/200 mg twice daily.
Clinically significant drug-drug interactions of APTIVUS co-administered with ritonavir are summarized in Table 4 below.
| Concomitant Drug Class: Drug name | Effect on Concentration of Tipranavir or Concomitant Drug | Clinical Comment |
|---|---|---|
| ↑ increase, ↓ decrease, ↔ no change, ↕ unable to predict | ||
| HIV-1 Antiviral Agents | ||
| Fusion Inhibitors: | ||
| Enfuvirtide | ↑ Tipranavir | At steady state, tipranavir trough concentrations were approximately 45% higher in patients co-administered enfuvirtide in the Phase 3 trials. The mechanism for this increase is not known. Dose adjustments are not recommended. |
| Non-Nucleoside Reverse Transcriptase Inhibitors: | ||
| Etravirine | ↓ Etravirine | APTIVUS/ritonavir when coadministered with etravirine may cause a significant decrease in the plasma concentrations of etravirine and loss of therapeutic effect of etravirine. Etravirine and APTIVUS/ritonavir should not be coadministered. |
| Rilpivirine | The use of rilpivirine co-administered with APTIVUS/ritonavir has not been studied. | Concomitant use of rilpivirine with Aptivus/ritonavir may cause an increase in the plasma concentrations of rilpivirine (inhibition of CYP3A enzymes). Rilpivirine is not expected to affect the plasma concentrations of Aptivus/ritonavir. |
| Nucleoside Reverse Transcriptase Inhibitors: | ||
| Abacavir | ↓ Abacavir AUC by approximately 40% | Clinical relevance of reduction in abacavir levels not established. Dose adjustment of abacavir cannot be recommended at this time. |
| Didanosine (EC) | ↓ Didanosine | Clinical relevance of reduction in didanosine levels not established. For optimal absorption, didanosine should be separated from APTIVUS/ritonavir dosing by at least 2 hours. |
| Zidovudine | ↓ Zidovudine AUC by approximately 35%. ZDV glucuronide concentrations were unaltered. | Clinical relevance of reduction in zidovudine levels not established. Dose adjustment of zidovudine cannot be recommended at this time. |
| Protease Inhibitors (co-administered with 200 mg of ritonavir): | ||
| Fosamprenavir | ↓ Amprenavir | Combining a protease inhibitor with APTIVUS/ritonavir is not recommended. |
| Lopinavir | ↓ Lopinavir | |
| Saquinavir | ↓ Saquinavir | |
| Protease Inhibitors (co-administered with 100 mg of ritonavir): | ||
| Atazanavir | ↓ Atazanavir | |
| ↑ Tipranavir | ||
| Virus Integrase Strand Transfer Inhibitors (INSTI): | ||
| Raltegravir | ↓ Raltegravir | No dose adjustment is needed for 400 mg twice daily dosing regimen of raltegravir. For all other dosing regimens of raltegravir, refer to current prescribing information for raltegravir. |
| Dolutegravir | ↓ Dolutegravir | For dosage recommendations, refer to dolutegravir prescribing information. |
| Agents for Opportunistic Infections | ||
| Antifungals: | ||
| Fluconazole Itraconazole Ketoconazole | ↑ Tipranavir, ↔ Fluconazole | Fluconazole increases tipranavir concentrations but dose adjustments are not needed. Fluconazole doses >200 mg/day are not recommended. |
| Voriconazole | ↑ Itraconazole (not studied) | |
| ↑ Ketoconazole (not studied) | Based on theoretical considerations itraconazole and ketoconazole should be used with caution. High doses (>200 mg/day) are not recommended. | |
| ↕ Voriconazole (not studied) | ||
| Due to multiple enzymes involved with voriconazole metabolism, it is difficult to predict the interaction. | ||
| Antimycobacterials: | ||
| Clarithromycin | ↑ Tipranavir, ↑ Clarithromycin, ↓ 14-hydroxy-clarithromycin metabolite | No dose adjustment of APTIVUS or clarithromycin for patients with normal renal function is necessary. |
For patients with renal impairment the following dosage adjustments should be considered:
| ||
| Rifabutin | Tipranavir not changed, ↑Rifabutin ↑ Desacetyl-rifabutin | Single dose study. Dosage reductions of rifabutin by 75% are recommended (e.g., 150 mg every other day). Increased monitoring for adverse events in patients receiving the combination is warranted. Further dosage reduction may be necessary. |
| Other Agents Commonly Used | ||
| Anticonvulsants: | ||
| Carbamazepine Phenobarbital Phenytoin | ↓ Tipranavir | Caution should be used when prescribing carbamazepine, phenobarbital and/or phenytoin. APTIVUS may be less effective due to decreased tipranavir plasma concentration in patients taking these agents concomitantly. |
| Valproic Acid | ↓ Valproic Acid | Caution should be used when prescribing valproic acid. Valproic acid may be less effective due to decreased valproic acid plasma concentration in patients taking APTIVUS concomitantly. |
| Antidepressants: | ||
| Trazodone | ↑ Trazodone | Concomitant use of trazodone and APTIVUS/ritonavir may increase plasma concentrations of trazodone. Adverse events of nausea, dizziness, hypotension, and syncope have been observed following co-administration of trazodone and ritonavir. If trazodone is used with a CYP 3A4 inhibitor such as APTIVUS/ritonavir, the combination should be used with caution and a lower dose of trazodone should be considered. |
| Desipramine | Combination with APTIVUS/ritonavir not studied ↑ Desipramine | Dosage reduction and concentration monitoring of desipramine is recommended. |
| Selective Serotonin-Reuptake Inhibitors: | Combination with APTIVUS/ritonavir not studied | Antidepressants have a wide therapeutic index, but doses may need to be adjusted upon initiation of APTIVUS/ritonavir therapy. |
| Fluoxetine Paroxetine Sertraline | ↑ Fluoxetine ↑ Paroxetine ↑ Sertraline | |
| Anti-gout | ||
| Colchicine | ↑ Colchicine | In patients with renal or hepatic impairment, coadministration of colchicine in patients on APTIVUS/ritonavir is contraindicated. |
| In combination with APTIVUS/ritonavir, the following dosage adjustments are recommended in patients with normal renal and hepatic function: | ||
Treatment of gout flares: Co-administration of colchicine in patients on APTIVUS/ritonavir:
| ||
Prophylaxis of gout flares: Co-administration of colchicine in patients on APTIVUS/ritonavir:
| ||
Treatment of familial Mediterranean fever (FMF): Co-administration of colchicine in patients on APTIVUS/ritonavir:
| ||
| Antipsychotics: | ||
| Quetiapine | ↑ Quetiapine | Initiation of APTIVUS with ritonavir in patients taking quetiapine: |
| Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. | ||
| Initiation of quetiapine in patients taking APTIVUS with ritonavir: | ||
| Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. | ||
| Benzodiazepines: | ||
| Parenterally administered midazolam | ↑ Midazolam | Midazolam is extensively metabolized by CYP 3A4. Increases in the concentration of midazolam are expected to be significantly higher with oral than parenteral administration. Therefore, APTIVUS should not be given with orally administered midazolam [see Contraindications (4) ]. If APTIVUS is co-administered with parenteral midazolam, close clinical monitoring for respiratory depression and/or prolonged sedation should be exercised and dosage adjustments should be considered. |
| Buprenorphine/naloxone | ↔ Buprenorphine ↓ Tipranavir | APTIVUS/ritonavir did not result in changes in the clinical efficacy of buprenorphine/naloxone. Compared to historical controls tipranavir Cmin was decreased approximately 40% with this combination. Dose adjustments cannot be recommended. |
| Calcium Channel Blockers: | Combination with APTIVUS/ritonavir not studied. Cannot predict effect of TPV/ritonavir on calcium channel blockers that are dual substrates of CYP3A and P-gp due to conflicting effect of TPV/ritonavir on CYP3A and P-gp. | Caution is warranted and clinical monitoring of patients is recommended. |
| Diltiazem Felodipine Nicardipine Nisoldipine Verapamil | ||
| ↕ Diltiazem ↑ Felodipine (CYP3A substrate but not P-gp substrate) ↕ Nicardipine ↕ Nisoldipine (CYP3A substrate but not clear whether it is a P-gp substrate) ↕ Verapamil | ||
| Disulfiram/Metronidazole | Combination with TPV/ritonavir not studied | APTIVUS capsules contain alcohol that can produce disulfiram-like reactions when co-administered with disulfiram or other drugs which produce this reaction (e.g., metronidazole). |
| Endothelin receptor antagonists | Co-administration of bosentan in patients on APTIVUS/ritonavir: | |
| Bosentan | ↑ Bosentan | In patients who have been receiving APTIVUS/ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability. |
| Co-administration of APTIVUS/ritonavir in patients on bosentan: | ||
| Discontinue use of bosentan at least 36 hours prior to initiation of APTIVUS/ritonavir. | ||
| After at least 10 days following the initiation of APTIVUS/ritonavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability. | ||
| HMG-CoA Reductase Inhibitors: | ||
| Atorvastatin Rosuvastatin | ↑ Atorvastatin ↓ Hydroxy-atorvastatin metabolites ↑ Rosuvastatin | Avoid co-administration with atorvastatin. |
| Hypoglycemics: | ||
| Combination with APTIVUS/ritonavir not studied | Careful glucose monitoring is warranted. | |
| Glimepiride Glipizide Glyburide Pioglitazone Repaglinide Tolbutamide | ↔ Glimepiride (CYP 2C9) ↔ Glipizide (CYP 2C9) ↔ Glyburide (CYP 2C9) ↕ Pioglitazone (CYP 2C8 and CYP 3A4) ↕ Repaglinide (CYP 2C8 and CYP 3A4) ↔ Tolbutamide (CYP 2C9) | |
| The effect of TPV/ritonavir on CYP 2C8 substrate is not known. | ||
| Immunosuppressants: | ||
| Combination with APTIVUS/ritonavir not studied. Cannot predict effect of TPV/ritonavir on immunosuppressants due to conflicting effect of TPV/ritonavir on CYP 3A and P-gp. | Increased frequency of monitoring of plasma levels of immunosuppressant drugs is recommended. | |
| Cyclosporine Sirolimus Tacrolimus | ↕ Cyclosporine ↕ Sirolimus ↕ Tacrolimus | |
| Inhaled beta agonist: | ||
| Salmeterol | ↑ Salmeterol | Concurrent administration of APTIVUS/ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
| Inhaled/Nasal Steroids: | ||
| Fluticasone | ↑ Fluticasone | Concomitant use of fluticasone propionate and APTIVUS/ritonavir may increase plasma concentrations of fluticasone propionate, resulting in significantly reduced serum cortisol concentrations. Co-administration of fluticasone propionate and APTIVUS/ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects. |
| Narcotic Analgesics: | ||
| Combinations with APTIVUS/ritonavir not studied | Dosage increase and long-term use of meperidine are not recommended due to increased concentrations of the metabolite normeperidine which has both analgesic activity and CNS stimulant activity (e.g., seizures). | |
| Meperidine | ↓ Meperidine, ↑ Normeperidine | |
| Methadone | ↓ Methadone | Dosage of methadone may need to be increased when co-administered with APTIVUS and 200 mg of ritonavir. |
| ↓ S-Methadone, ↓ R-Methadone | ||
| Oral Contraceptives/Estrogens: | ||
| Ethinyl estradiol | ↓ Ethinyl estradiol concentrations by 50% | Alternative methods of nonhormonal contraception should be used when estrogen based oral contraceptives are co-administered with APTIVUS and 200 mg of ritonavir. Patients using estrogens as hormone replacement therapy should be clinically monitored for signs of estrogen deficiency. Women using estrogens may have an increased risk of non-serious rash. |
| Proton Pump Inhibitors: | ||
| Omeprazole | ↓ Omeprazole, ↔ Tipranavir | Dosage of omeprazole may need to be increased when co-administered with APTIVUS and ritonavir. |
| PDE-5 Inhibitors: | ||
| Only the combination of tadalafil with APTIVUS/ritonavir has been studied (at doses used for treatment of erectile dysfunction). | Co-administration with APTIVUS/ritonavir may result in an increase in PDE-5 inhibitor-associated adverse events, including hypotension, syncope, visual disturbances, and priapism. | |
| Sildenafil Tadalafil Vardenafil | ↑ Sildenafil (not studied) ↑ Tadalafil with first dose APTIVUS/ritonavir ↔ Tadalafil at APTIVUS/ritonavir steady-state ↑ Vardenafil (not studied) | Use of PDE-5 inhibitors for pulmonary arterial hypertension (PAH):
|
| Co-administration of tadalafil (Adcirca) in patients on APTIVUS/ritonavir: | ||
| In patients receiving APTIVUS/ritonavir for at least one week, start Adcirca at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. | ||
| Co-administration of APTIVUS/ritonavir in patients on tadalafil (Adcirca): | ||
| Avoid use of tadalafil (Adcirca) during the initiation of APTIVUS/ritonavir. Stop Adcirca at least 24 hours prior to starting APTIVUS/ritonavir. After at least one week following the initiation of APTIVUS/ritonavir, resume Adcirca at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. | ||
| Use of PDE-5 inhibitors for erectile dysfunction: | ||
Concomitant use of PDE-5 inhibitors with APTIVUS/ritonavir should be used with caution and in no case should the starting dose of:
| ||
| Use with increased monitoring for adverse events. | ||
| Oral Anticoagulants: | ||
| Warfarin | ↔ S-Warfarin | Frequent INR (international normalized ratio) monitoring upon initiation of APTIVUS/ritonavir therapy. |
8. Use in Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to APTIVUS during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Prospective pregnancy data from the APR and an Expanded Access program are not sufficient to adequately assess the risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Tipranavir use during pregnancy has been evaluated in a limited number of women as reported by the APR and an Expanded Access program, and available data show no birth defects in 13 first trimester exposures (see Data) compared with the background rate for major birth defects of 2.7% in the US reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15-20%. The background risk of birth defects and miscarriage for the indicated population is unknown. Methodological limitations of the APR include the use of MACDP as the external comparator group. The MACDP population is not disease-specific, evaluates women and infants from a limited geographic area, and does not include outcomes for births that occurred at <20 weeks gestation.
In animal reproduction studies, fetal toxicities were observed with tipranavir at maternally toxic doses with systemic exposures (AUC) less than those in humans at the recommended human dose (RHD) (see Data).
Data
Human Data
Based on prospective reports to the APR and an Expanded Access program for approximately 17 live births following exposure to tipranavir-containing regimens (including 13 live births exposed in the first trimester and 4 live births exposed in the second/third trimester), there were no birth defects reported in live-born infants.
Tipranavir has been shown to cross the placenta.
Animal Data
Tipranavir was administered orally to pregnant rats (at 0, 40, 400, or 1000 mg/kg/day from gestation day 6 to 17) and rabbits (at 0, 75, 150, or 375 mg/kg/day from gestation day 6 to 20). In rats, fetal toxicities including decreased body weight and sternebrae ossification occurred at maternally toxic doses (≥400 mg/kg/day) (approximately 0.8 times human exposure at the RHD). In rabbits, fetal toxicities including decreased fetal body weights, wavy ribs, and bent femurs occurred at a maternally toxic dose (375 mg/kg/day) (approximately 0.05 times human exposure at the RHD). Maternal toxicity included an increased incidence of abortions at doses ≥150 mg/kg/day (approximately 0.05 times human exposure at the RHD).
In the pre/post-natal development study, tipranavir was administered orally to rats at 0, 40, 400, 1000 mg/kg/day from gestation day 6 to lactation day 21. The only significant effect observed was growth inhibition of the offspring at maternally toxic doses (≥400 mg/kg/day) (approximately 0.8 times human exposure at the RHD).
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers in the United States not breast-feed their infants to avoid risking postnatal transmission of HIV-1 infection. There is no information regarding the presence of tipranavir in human milk, the effects on the breastfed infant, or the effects on milk production. Tipranavir is present in rat milk (see Data). Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive patients), and (3) any possible adverse effects of APTIVUS, mothers should not breastfeed if they are receiving APTIVUS.
Data
In a lactation study, tipranavir was excreted into the milk of lactating rats following a single oral dose of tipranavir (10 mg/kg) on lactation/postpartum day 14, with a maximal milk concentration achieved 2 hours post-administration (milk concentration 0.13 times that of maternal plasma concentration).
8.3 Females and Males of Reproductive Potential
Contraception
Use of APTIVUS may reduce the efficacy of estrogen-based oral contraceptives. Advise patients to use alternative methods of nonhormonal contraception [see Drug Interactions (7.2) ].
8.4 Pediatric Use
The safety, pharmacokinetic profile, and virologic and immunologic responses of APTIVUS oral solution and capsules were evaluated in HIV-1 infected pediatric patients age 2 to 18 years [see Adverse Reactions (6.2) and Clinical Studies (14.2) ].
The most frequent adverse reactions (grades 2-4) were similar to those described in adults. However, rash was reported more frequently in pediatric patients than in adults [see Warnings and Precautions (5.6) and Adverse Reactions (6.2) ].
The risk-benefit has not been established in pediatric patients <2 years of age.
8.5 Geriatric Use
Clinical studies of APTIVUS/ritonavir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. In general, caution should be exercised in the administration and monitoring of APTIVUS in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Tipranavir is principally metabolized by the liver. Caution should be exercised when administering APTIVUS/ritonavir to patients with mild (Child-Pugh Class A) hepatic impairment because tipranavir concentrations may be increased [see Clinical Pharmacology (12.3) ]. APTIVUS/ritonavir is contraindicated in patients with moderate or severe (Child-Pugh Class B or Child-Pugh Class C) hepatic impairment [see Contraindications (4) ].
10. Overdosage
There is no known antidote for APTIVUS overdose. Treatment of overdose should consist of general supportive measures, including monitoring of vital signs and observation of the patient's clinical status. If indicated, elimination of unabsorbed tipranavir should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since tipranavir is highly protein bound, dialysis is unlikely to provide significant removal of the drug.
11. Description
APTIVUS is a protease inhibitor of HIV-1 belonging to the class of 4-hydroxy-5,6-dihydro-2-pyrone sulfonamides.
The chemical name of tipranavir is 2-Pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl). It has a molecular formula of C31H33F3N2O5S and a molecular weight of 602.7. Tipranavir has the following structural formula and is a single stereoisomer with the 1R, 6R configuration.
Tipranavir is a white to off-white to slightly yellow solid. It is freely soluble in dehydrated alcohol and propylene glycol, and insoluble in aqueous buffer at pH 7.5.
APTIVUS soft gelatin capsules are for oral administration. Each capsule contains 250 mg tipranavir. The major inactive ingredients in the capsule are dehydrated alcohol (7% w/w or 0.1 g per capsule), polyoxyl 35 castor oil, propylene glycol, mono/diglycerides of caprylic/capric acid and gelatin.
APTIVUS oral solution is available in a strength of 100 mg/mL of tipranavir. APTIVUS oral solution is a yellow, viscous clear liquid with a buttermint-butter toffee flavor. The major inactive ingredients in the oral solution are polyethylene glycol 400, vitamin E polyethylene glycol succinate (TPGS), purified water, and propylene glycol. Each milliliter of APTIVUS oral solution contains 116 IU of vitamin E, and when taken at the recommended maximum dose of 500 mg/200 mg tipranavir/ritonavir BID results in a daily dose of 1160 IU.
12. Clinical Pharmacology
12.2 Pharmacodynamics
ECG Evaluation
The effect of APTIVUS/ritonavir on the QTcF interval was measured in a study in which 81 healthy subjects received the following treatments twice daily for 2.5 days: APTIVUS/ritonavir (500/200 mg), APTIVUS/ritonavir at a supra-therapeutic dose (750/200 mg), and placebo/ritonavir (-/200 mg). After baseline and placebo adjustment, the maximum mean QTcF change was 3.2 ms (1-sided 95% Upper CI: 5.6 ms) for the 500/200 mg dose and 8.3 ms (1-sided 95% Upper CI: 10.9 ms) for the supra-therapeutic 750/200 mg dose.
Antiviral Activity in vivo
The median Inhibitory Quotient (IQ) determined from 264 treatment-experienced adult patients was about 80 (inter-quartile range: 31-226), from the controlled clinical trials 1182.12 and 1182.48. The IQ is defined as the tipranavir trough concentration divided by the viral EC50 value, corrected for protein binding. There was a relationship between the proportion of patients with a ≥1 log10 reduction of viral load from baseline at week 48 and their IQ value. Among the 198 patients receiving APTIVUS/ritonavir with no new enfuvirtide use (e.g., new enfuvirtide, defined as initiation of enfuvirtide for the first time), the response rate was 23% in those with an IQ value <80 and 59% in those with an IQ value ≥80. Among the 66 patients receiving APTIVUS/ritonavir with new enfuvirtide, the response rates in patients with an IQ value <80 versus those with an IQ value ≥80 were 55% and 71%, respectively. These IQ groups are derived from a select population and are not meant to represent clinical breakpoints.
12.3 Pharmacokinetics
In order to achieve effective tipranavir plasma concentrations and a twice-daily dosing regimen, co-administration of APTIVUS with ritonavir is essential [see Dosage and Administration (2) ]. Ritonavir inhibits hepatic cytochrome P450 3A (CYP 3A), the intestinal P-gp efflux pump and possibly intestinal CYP 3A. In a dose-ranging evaluation in 113 HIV-1 negative male and female volunteers, there was a 29-fold increase in the geometric mean morning steady-state trough plasma concentrations of tipranavir following APTIVUS co-administered with low-dose ritonavir (500/200 mg twice daily) compared to APTIVUS 500 mg twice daily without ritonavir. In adults the mean systemic ritonavir concentration when 200 mg of ritonavir was given with 500 mg of APTIVUS was similar to the concentrations observed when 100 mg was given with the other protease inhibitors.
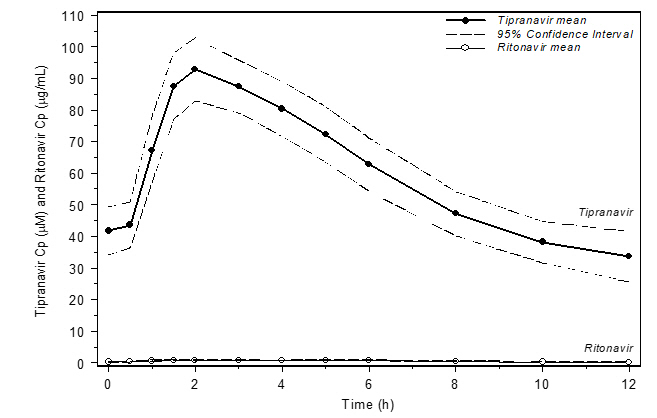
Figure 1 displays mean plasma concentrations of tipranavir and ritonavir at steady state for 30 HIV-1 infected adult patients dosed with 500/200 mg tipranavir/ritonavir for 14 days.
Figure 1 Mean Steady State Tipranavir Plasma Concentrations (95% CI) with Ritonavir Co-administration (tipranavir/ritonavir 500/200 mg BID)

Absorption and Bioavailability
Absorption of tipranavir in humans is limited, although no absolute quantification of absorption is available. Tipranavir is a P-gp substrate, a weak P-gp inhibitor, and appears to be a potent P-gp inducer as well. In vivo data suggest that tipranavir/ritonavir, at the dose of 500/200 mg, is a P-gp inhibitor after the first dose and induction of P-gp occurs over time. Tipranavir trough concentrations at steady-state are about 70% lower than those on Day 1, presumably due to intestinal P-gp induction. Steady state is attained in most subjects after 7-10 days of dosing.
Dosing APTIVUS 500 mg with 200 mg ritonavir capsules twice daily for greater than 2 weeks and without meal restriction produced the pharmacokinetic parameters for male and female HIV-1 positive patients presented in Table 5.
| Parameter | Females (n=14) | Males (n=106) |
|---|---|---|
| aPopulation pharmacokinetic parameters reported as mean ± standard deviation | ||
| Cptrough (µM) | 41.6 ± 24.3 | 35.6 ± 16.7 |
| Cmax (µM) | 94.8 ± 22.8 | 77.6 ± 16.6 |
| Tmax (h) | 2.9 | 3.0 |
| AUC0-12h (µM∙h) | 851 ± 309 | 710 ± 207 |
| CL (L/h) | 1.15 | 1.27 |
| V (L) | 7.7 | 10.2 |
| t1/2 (h) | 5.5 | 6.0 |
Effects of Food on Oral Absorption
For APTIVUS capsules or oral solution co-administered with ritonavir capsules at steady-state, no clinically significant changes in tipranavir Cmax, Cp12h, and AUC were observed under fed conditions (500-682 Kcal, 23-25% calories from fat) compared to fasted conditions [see Dosage and Administration (2) ]. The effect of food on tipranavir exposure when APTIVUS capsules or oral solution is co-administered with ritonavir tablets has not been evaluated [see Dosage and Administration (2) ]. For information on the effect of food on the bioavailability of ritonavir tablets, please refer to the ritonavir tablet prescribing information.
Distribution
Tipranavir is extensively bound to plasma proteins (>99.9%). It binds to both human serum albumin and α-1-acid glycoprotein. The mean fraction of tipranavir (dosed without ritonavir) unbound in plasma was similar in clinical samples from healthy volunteers and HIV-1 positive patients. Total plasma tipranavir concentrations for these samples ranged from 9 to 82 µM. The unbound fraction of tipranavir appeared to be independent of total drug concentration over this concentration range.
No studies have been conducted to determine the distribution of tipranavir into human cerebrospinal fluid or semen.
Metabolism
In vitro metabolism studies with human liver microsomes indicated that CYP 3A4 is the predominant CYP enzyme involved in tipranavir metabolism.
The oral clearance of tipranavir decreased after the addition of ritonavir, which may represent diminished first-pass clearance of the drug at the gastrointestinal tract as well as the liver.
The metabolism of tipranavir in the presence of 200 mg ritonavir is minimal. Administration of 14C-tipranavir to subjects that received APTIVUS/ritonavir 500/200 mg dosed to steady-state demonstrated that unchanged tipranavir accounted for 98.4% or greater of the total plasma radioactivity circulating at 3, 8, or 12 hours after dosing. Only a few metabolites were found in plasma, and all were at trace levels (0.2% or less of the plasma radioactivity). In feces, unchanged tipranavir represented the majority of fecal radioactivity (79.9% of fecal radioactivity). The most abundant fecal metabolite, at 4.9% of fecal radioactivity (3.2% of dose), was a hydroxyl metabolite of tipranavir. In urine, unchanged tipranavir was found in trace amounts (0.5% of urine radioactivity). The most abundant urinary metabolite, at 11.0% of urine radioactivity (0.5% of dose) was a glucuronide conjugate of tipranavir.
Elimination
Administration of 14C-tipranavir to subjects (n=8) that received APTIVUS/ritonavir 500/200 mg dosed to steady-state demonstrated that most radioactivity (median 82.3%) was excreted in feces, while only a median of 4.4% of the radioactive dose administered was recovered in urine. In addition, most radioactivity (56%) was excreted between 24 and 96 hours after dosing. The effective mean elimination half-life of tipranavir/ritonavir in healthy volunteers (n=67) and HIV-1 infected adult patients (n=120) was approximately 4.8 and 6.0 hours, respectively, at steady state following a dose of 500/200 mg twice daily with a light meal.
Special Populations
Renal Impairment
APTIVUS pharmacokinetics has not been studied in patients with renal dysfunction. However, since the renal clearance of tipranavir is negligible, a decrease in total body clearance is not expected in patients with renal insufficiency.
Hepatic Impairment
In a study comparing 9 HIV-1 negative patients with mild (Child-Pugh Class A) hepatic impairment to 9 HIV-1 negative controls, the single and multiple dose plasma concentrations of tipranavir and ritonavir were increased in patients with hepatic impairment, but were within the range observed in clinical trials. No dosing adjustment is required in patients with mild hepatic impairment.
The influence of moderate hepatic impairment (Child-Pugh Class B) or severe hepatic impairment (Child-Pugh Class C) on the multiple-dose pharmacokinetics of tipranavir administered with ritonavir has not been evaluated [see Dosage and Administration (2), Contraindications (4), and Warnings and Precautions (5.1) ].
Gender
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that females generally had higher tipranavir concentrations than males. After 4 weeks of APTIVUS/ritonavir 500/200 mg BID, the median plasma trough concentration of tipranavir was 43.9 µM for females and 31.1 µM for males. The difference in concentrations does not warrant a dose adjustment.
Race
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that white males generally had more variability in tipranavir concentrations than black males, but the median concentration and the range making up the majority of the data are comparable between the races.
Geriatric Patients
Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that there was no change in median trough tipranavir concentrations as age increased for either gender through 65 years of age. There were an insufficient number of women greater than age 65 years in the two trials to evaluate the elderly.
Pediatric Patients
Among pediatric patients in clinical trial 1182.14, steady-state plasma tipranavir trough concentrations were obtained 10 to 14 hours following study drug administration. Pharmacokinetic parameters by age group are presented in Table 6.
| Parameter | 2 to <6 years (n=12) | 6 to <12 years (n=8) | 12 to 18 years (n=6) |
|---|---|---|---|
| aPopulation pharmacokinetic parameters reported as mean ± standard deviation | |||
| Cptrough (µM) | 59.6 ± 23.6 | 66.3 ± 12.5 | 53.3 ± 32.4 |
| Cmax (µM) | 135 ± 44 | 151 ± 32 | 138 ± 52 |
| Tmax (h) | 2.5 | 2.6 | 2.7 |
| AUC0-12h (µM∙h) | 1190 ± 332 | 1354 ± 256 | 1194 ± 517 |
| CL/F (L/h) | 0.34 | 0.45 | 0.99 |
| V (L) | 4.0 | 4.7 | 5.3 |
| t1/2 (h) | 8.1 | 7.1 | 5.2 |
Drug Interactions
Drug interaction studies were performed with APTIVUS capsules co-administered with ritonavir, and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of APTIVUS with 200 mg ritonavir on the AUC, Cmax, and Cmin of tipranavir or the co-administered drug, are summarized in Tables 7 and 8, respectively. For information regarding clinical recommendations see Drug Interactions (7.2).
| Co-administered Drug | Co-administered Drug Dose (Schedule) | tipranavir/ ritonavir Drug Dose (Schedule) | n | PK | Ratio (90% Confidence Interval) of Tipranavir Pharmacokinetic Parameters with/without Co-administered Drug; No Effect = 1.00 | ||
|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | |||||
| *steady state comparison to historical data (n) | |||||||
| ↑ increase, ↓ decrease, ↔ no change, ↕ unable to predict | |||||||
| Antacids (Maalox®) | 20 mL (1 dose) | 500/200 mg (1 dose) | 23 | ↓ | 0.75 (0.63, 0.88) | 0.73 (0.64, 0.84) | - |
| Atazanavir/ritonavir | 300/100 mg QD (9 doses) | 500/100 mg BID (34 doses) | 13 | ↑ | 1.08 (0.98, 1.20) | 1.20 (1.09, 1.32) | 1.75 (1.39, 2.20) |
| Atorvastatin | 10 mg (1 dose) | 500/200 mg BID (14 doses) | 22 | ↔ | 0.96 (0.86, 1.07) | 1.08 (1.00, 1.15) | 1.04 (0.89, 1.22) |
| Clarithromycin | 500 mg BID (25 doses) | 500/200 mg BID* | 24 (68) | ↑ | 1.40 (1.24, 1.47) | 1.66 (1.43, 1.73) | 2.00 (1.58, 2.47) |
| Didanosine | 400 mg (1 dose) | 500/100 mg BID (27 doses) | 5 | ↓ | 1.32 (1.09, 1.60) | 1.08 (0.82, 1.42) | 0.66 (0.31, 1.43) |
| Efavirenz | 600 mg QD (8 doses) | 500/100 mg BID* | 21 (89) | ↓ | 0.79 (0.69, 0.89) | 0.69 (0.57, 0.83) | 0.58 (0.36, 0.86) |
| 750/200 mg BID* | 25 (100) | ↔ | 0.97 (0.85, 1.09) | 1.01 (0.85, 1.18) | 0.97 (0.69, 1.28) | ||
| Ethinyl estradiol /Norethindrone | 0.035/1.0 mg (1 dose) | 500/100 mg BID (21 doses) | 21 | ↓ | 1.10 (0.98, 1.24) | 0.98 (0.88, 1.11) | 0.73 (0.59, 0.90) |
| 750/200 mg BID (21 doses) | 13 | ↔ | 1.01 (0.96, 1.06) | 0.98 (0.90, 1.07) | 0.91 (0.69, 1.20) | ||
| Fluconazole | 100 mg QD (12 doses) | 500/200 mg BID* | 20 (68) | ↑ | 1.32 (1.18, 1.47) | 1.50 (1.29, 1.73) | 1.69 (1.33, 2.09) |
| Loperamide | 16 mg (1 dose) | 750/200 mg BID (21 doses) | 24 | ↓ | 1.03 (0.92, 1.17) | 0.98 (0.86, 1.12) | 0.74 (0.62, 0.88) |
| Rifabutin | 150 mg (1 dose) | 500/200 mg BID (15 doses) | 21 | ↔ | 0.99 (0.93, 1.07) | 1.00 (0.96, 1.04) | 1.16 (1.07, 1.27) |
| Rosuvastatin | 10 mg (1 dose) | 500/200 mg BID (24 doses) | 16 | ↔ | 1.08 (1.00, 1.17) | 1.06 (0.97, 1.15) | 0.99 (0.88, 1.11) |
| Tadalafil | 10 mg (1 dose) | 500/200 mg BID (17 doses) | 17 | ↔ | 0.90 (0.80, 1.01) | 0.85 (0.74, 0.97) | 0.81 (0.70, 0.94) |
| Tenofovir | 300 mg (1 dose) | 500/100 mg BID | 22 | ↓ | 0.83 (0.74, 0.94) | 0.82 (0.75, 0.91) | 0.79 (0.70, 0.90) |
| 750/200 mg BID (23 doses) | 20 | ↔ | 0.89 (0.84, 0.96) | 0.91 (0.85, 0.97) | 0.88 (0.78, 1.00) | ||
| Valacyclovir | 500 mg (1 dose) | 500/200 mg BID (23 doses) | 26 | ↔ | 1.02 (0.95, 1.10) | 1.01 (0.96, 1.06) | 0.98 (0.93, 1.04) |
| Zidovudine | 300 mg (1 dose) | 500/100 mg BID | 29 | ↓ | 0.87 (0.80, 0.94) | 0.82 (0.76, 0.89) | 0.77 (0.68, 0.87) |
| 750/200 mg BID (23 doses) | 25 | ↔ | 1.02 (0.94, 1.10) | 1.02 (0.92, 1.13) | 1.07 (0.86, 1.34) | ||
| Co-administered Drug | Co-administered Drug Dose (Schedule) | tipranavir/ritonavir Drug Dose (Schedule) | n | PK | Ratio (90% Confidence Interval) of Co-administered Drug Pharmacokinetic Parameters with/without tipranavir/ritonavir; No Effect = 1.00 | ||
|---|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | |||||
| a HIV-1 positive patients | |||||||
| b Buprenorphine/Naloxone maintenance patients | |||||||
| c HIV-1 positive patients (tipranavir/ritonavir 250 mg/200 mg, 750 mg/200 mg and 1250 mg/100 mg) and healthy volunteers (tipranavir/ritonavir 500 mg/100 mg and 750 mg/200 mg) | |||||||
| d Normalized sum of parent drug (rifabutin) and active metabolite (25-O-desacetyl-rifabutin) | |||||||
| e Intensive PK analysis | |||||||
| f Drug levels obtained at 8-16 hrs post-dose | |||||||
| g n = 14 for Cmin | |||||||
| hAdministered as Valacyclovir | |||||||
| ↑ increase, ↓ decrease, ↔ no change, ↕ unable to predict | |||||||
| Abacavira | 300 mg BID (43 doses) | 250/200 mg BID | 28 | ↓ | 0.56 (0.48, 0.66) | 0.56 (0.49, 0.63) | - |
| 750/100 mg BID | 14 | ↓ | 0.54 (0.47, 0.63) | 0.64 (0.55, 0.74) | - | ||
| 1250/100 mg BID (42 doses) | 11 | ↓ | 0.48 (0.42, 0.53) | 0.65 (0.55, 0.76) | - | ||
| Acyclovirh | 500 mg (1 dose) | 500/200 mg BID (23 doses) | 26 | ↔ | 0.95 (0.88, 1.02) | 1.07 (1.04, 1.09) | - |
| Amprenavir/ritonavira | 600/100 mg BID (27 doses) | 500/200 mg BID (28 doses) | 16 74 | ↓ ↓ | 0.61 (0.51, 0.73)e | 0.56 (0.49, 0.64)e
- | 0.45 (0.38, 0.53)e
0.44 (0.39, 0.49)f |
| Atazanavir/ritonavir | 300/100 mg QD (9 doses) | 500/100 mg BID (34 doses) | 13 | ↓ | 0.43 (0.38, 0.50) | 0.32 (0.29, 0.36) | 0.19 (0.15, 0.24) |
| Atorvastatin | 10 mg (1 dose) | 500/200 mg BID (17 doses) | 22 | ↑ | 8.61 (7.25, 10.21) | 9.36 (8.02, 10.94) | 5.19 (4.21, 6.40) |
| Orthohydroxy-atorvastatin | 21, 12, 17 | ↓ | 0.02 (0.02, 0.03) | 0.11 (0.08, 0.17) | 0.07 (0.06, 0.08) | ||
| Parahydroxy-atorvastatin | 13, 22, 1 | ↓ | 1.04 (0.87, 1.25) | 0.18 (0.14, 0.24) | 0.33 (NA) | ||
| Buprenorphine/ Naloxoneb | 16/4 mg 24/6 mg (daily) | 500/200 mg BID (16 doses) | |||||
| Buprenorphine | 10 | ↔ | 0.86 (0.68, 1.10) | 0.99 (0.80, 1.23) | 0.94 (0.74, 1.19) | ||
| Carbamazepine | 100 mg BID (29 doses) | 500/200 mg (1 dose) | 7 | ↔ | 1.04 (1.00, 1.07) | 1.05 (1.02, 1.09) | 1.17 (1.11, 1.24) |
| (43 doses) | (15 doses) | 7 | ↔ | 1.10 (0.85, 1.42) | 1.08 (0.91, 1.27) | 1.07 (0.90, 1.27) | |
| 200 mg BID (29 doses) | 500/200 mg (1 dose) | 17 | ↔ | 1.00 (0.96, 1.04) | 1.04 (1.00, 1.08) | 1.16 (1.11, 1.22) | |
| (43 doses) | (15 doses) | 17 | ↑ | 1.22 (1.11, 1.34) | 1.26 (1.15, 1.38) | 1.35 (1.22, 1.50) | |
| Clarithromycin | 500 mg BID (25 doses) | 500/200 mg BID (15 doses) | 21 | ↑ | 0.95 (0.83, 1.09) | 1.19 (1.04, 1.37) | 1.68 (1.42, 1.98) |
| 14-OH-clarithromycin | 21 | ↓ | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.05 (0.04, 0.07) | ||
| Didanosinec | 200 mg BID, ≥60 kg | 250/200 mg BID | 10 | ↓ | 0.57 (0.42, 0.79) | 0.67 (0.51, 0.88) | - |
| 125 mg BID, <60 kg (43 doses) | 750/100 mg BID | 8 | ↔ | 0.76 (0.49, 1.17) | 0.97 (0.64, 1.47) | - | |
| 1250/100 mg BID (42 doses) | 9 | ↔ | 0.77 (0.47, 1.26) | 0.87 (0.47, 1.65) | - | ||
| 400 mg (1 dose) | 500/100 mg BID (27 doses) | 5 | ↔ | 0.80 (0.63, 1.02) | 0.90 (0.72, 1.11) | 1.17 (0.62, 2.20) | |
| Dolutegravir | 50 mg QD | 500/200 mg BID | 14 | ↓ | 0.54 (0.50-0.57) | 0.4 (0.38-0.44) | 0.24(0.21-0.27) |
| Efavirenzc | 600 mg QD (15 doses) | 500/100 mg BID | 24 | ↔ | 1.09 (0.99, 1.19) | 1.04 (0.97, 1.12) | 1.02 (0.92, 1.12) |
| 750/200 mg BID (15 doses) | 22 | ↔ | 1.12 (0.98, 1.28) | 1.00 (0.93, 1.09) | 0.94 (0.84, 1.04) | ||
| Ethinyl estradiol | 0.035 mg (1 dose) | 500/100 mg BID | 21 | ↓ | 0.52 (0.47, 0.57) | 0.52 (0.48, 0.56) | - |
| 750/200 mg BID (21 doses) | 13 | ↓ | 0.48 (0.42, 0.57) | 0.57 (0.54, 0.60) | - | ||
| Fluconazole | 200 mg (Day 1) then 100 mg QD (6 or 12 doses) | 500/200 mg BID (2 or 14 doses) | 19 | ↔ | 0.97 (0.94, 1.01) | 0.99 (0.97, 1.02) | 0.98 (0.94, 1.02) |
| 19 | ↔ | 0.94 (0.91, 0.98) | 0.92 (0.88, 0.95) | 0.89 (0.85, 0.92) | |||
| Lopinavir/ritonavira | 400/100 mg BID (27 doses) | 500/200 mg BID (28 doses) | 21 | ↓ | 0.53 (0.40, 0.69)e | 0.45 (0.32, 0.63)e | 0.30 (0.17, 0.51)e |
| 69 | ↓ | - | - | 0.48 (0.40, 0.58)f | |||
| Loperamide | 16 mg (1 dose) | 750/200 mg BID (21 doses) | 24 | ↓ | 0.39 (0.31, 0.48) | 0.49 (0.40, 0.61) | - |
| N-Demethyl-Loperamide | 24 | ↓ | 0.21 (0.17, 0.25) | 0.23 (0.19, 0.27) | - | ||
| Lamivudinea | 150 mg BID (43 doses) | 250/200 mg BID 750/100 mg BID 1250/100 mg BID (42 doses) | 64 46 35 | ↔ ↔ ↔ | 0.96 (0.89, 1.03) 0.86 (0.78, 0.94) 0.71 (0.62, 0.81) | 0.95 (0.89, 1.02) 0.96 (0.90, 1.03) 0.82 (0.66, 1.00) | - - - |
| Methadone | 5 mg (1 dose) | 500/200 mg BID (16 doses) | 14 | ↓ | 0.45 (0.41, 0.49) | 0.47 (0.44, 0.51) | 0.50 (0.46, 0.54) |
| R-methadone | 0.54 (0.50, 0.58) | 0.52 (0.49, 0.56) | - | ||||
| S-methadone | 0.38 (0.35, 0.43) | 0.37 (0.34, 0.41) | - | ||||
| Nevirapinea | 200 mg BID (43 doses) | 250/200 mg BID 750/100 mg BID 1250/100 mg BID (42 doses) | 26 22 17 | ↔ ↔ ↔ | 0.97 (0.90, 1.04) 0.86 (0.76, 0.97) 0.71 (0.62, 0.82) | 0.97 (0.91, 1.04) 0.89 (0.78, 1.01) 0.76 (0.63, 0.91) | 0.96 (0.87, 1.05) 0.93 (0.80, 1.08) 0.77 (0.64, 0.92) |
| Norethindrone | 1.0 mg (1 dose) | 500/100 mg BID 750/200 mg BID (21 doses) | 21 13 | ↔ ↔ | 1.03 (0.94, 1.13) 1.08 (0.97, 1.20) | 1.14 (1.06, 1.22) 1.27 (1.13, 1.43) | - - |
| Raltegravir | 400 mg BID | 500/200 mg BID | 15 | ↓ | 0.82 (0.46, 1.46) | 0.76 (0.49, 1.19) | 0.45 (0.31, 0.66)g |
| Rifabutin | 150 mg (1 dose) | 500/200 mg BID (15 doses) | 20 | ↑ | 1.70 (1.49, 1.94) | 2.90 (2.59, 3.26) | 2.14 (1.90, 2.41) |
| 25-O-desacetyl-rifabutin | 20 | ↑ | 3.20 (2.78, 3.68) | 20.71 (17.66, 24.28) | 7.83 (6.70, 9.14) | ||
| Rifabutin + 25-O-desacetyl-rifabutind | 20 | ↑ | 1.86 (1.63, 2.12) | 4.33 (3.86, 4.86) | 2.76 (2.44, 3.12) | ||
| Rosuvastatin | 10 mg (1 dose) | 500/200 mg BID (24 doses) | 16 | ↑ | 2.23 (1.83, 2.72) | 1.26 (1.08, 1.46) | 1.06 (0.93, 1.20) |
| Saquinavir/ritonavira | 600/100 mg BID (27 doses) | 500/200 mg BID (28 doses) | 20 68 | ↓ ↓ | 0.30 (0.23, 0.40)e
- | 0.24 (0.19, 0.32)e
- | 0.18 (0.13, 0.26)e
0.20 (0.16, 0.25)f |
| Stavudinea | 40 mg BID ≥60 kg | 250/200 mg BID | 26 | ↔ | 0.90 (0.81, 1.02) | 1.00 (0.91, 1.11) | - |
| 30 mg BID <60 kg (43 doses) | 750/100 mg BID | 22 | ↔ | 0.76 (0.66, 0.89) | 0.84 (0.74, 0.96) | - | |
| 1250/100 mg BID (42 doses) | 19 | ↔ | 0.74 (0.69, 0.80) | 0.93 (0.83, 1.05) | - | ||
| Tadalafil | 10 mg (1 dose) | 500/200 mg (1 dose) | 17 | ↑ | 0.78 (0.72, 0.84) | 2.33 (2.02, 2.69) | - |
| 10 mg (1 dose) | 500/200 mg BID (17 doses) | 17 | ↔ | 0.70 (0.63, 0.78) | 1.01 (0.83, 1.21) | - | |
| Tenofovir | 300 mg (1 dose) | 500/100 mg BID 750/200 mg BID (23 doses) | 22 20 | ↓ ↓ | 0.77 (0.68, 0.87) 0.62 (0.54, 0.71) | 0.98 (0.91, 1.05) 1.02 (0.94, 1.10) | 1.07 (0.98, 1.17) 1.14 (1.01, 1.27) |
| Zidovudinec | 300 mg BID | 250/200 mg BID | 48 | ↓ | 0.54 (0.47, 0.62) | 0.58 (0.51, 0.66) | - |
| 300 mg BID | 750/100 mg BID | 31 | ↓ | 0.51 (0.44, 0.60) | 0.64 (0.55, 0.75) | - | |
| 300 mg BID (43 doses) | 1250/100 mg BID (42 doses) | 23 | ↓ | 0.49 (0.40, 0.59) | 0.69 (0.49, 0.97) | - | |
| 300 mg (1 dose) | 500/100 mg BID | 29 | ↓ | 0.39 (0.33, 0.45) | 0.57 (0.52, 0.63) | 0.89 (0.81, 0.99) | |
| 750/200 mg BID (23 doses) | 25 | ↔ | 0.44 (0.36, 0.54) | 0.67 (0.62, 0.73) | 1.25 (1.08, 1.44) | ||
| Zidovudine glucuronide | 500/100 mg BID | 29 | ↑ | 0.82 (0.74, 0.90) | 1.02 (0.97, 1.06) | 1.52 (1.34, 1.71) | |
| 750/200 mg BID (23 doses) | 25 | ↑ | 0.82 (0.73, 0.92) | 1.09 (1.05, 1.14) | 1.94 (1.62, 2.31) | ||
12.4 Microbiology
Mechanism of Action
Tipranavir (TPV) is an HIV-1 protease inhibitor that inhibits the virus-specific processing of the viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.
Antiviral Activity
Tipranavir inhibits the replication of laboratory strains of HIV-1 and clinical isolates in acute models of T-cell infection, with 50% effective concentrations (EC50) ranging from 0.03 to 0.07 µM (18-42 ng/mL). Tipranavir demonstrates antiviral activity in cell culture against a broad panel of HIV-1 group M non-clade B isolates (A, C, D, F, G, H, CRF01 AE, CRF02 AG, CRF12 BF). Group O and HIV-2 isolates have reduced susceptibility in cell culture to tipranavir with EC50 values ranging from 0.164 -1 µM and 0.233-0.522 µM, respectively. The cell culture antiviral activity of tipranavir in combination with the HIV-1 protease inhibitors amprenavir, atazanavir, lopinavir and saquinavir, and with the HIV-1 NRTI lamivudine was additive to antagonistic. No antagonism was seen when combined with the HIV-1 protease inhibitors indinavir, nelfinavir, or ritonavir, with the NNRTIs delavirdine, efavirenz, and nevirapine, with the NRTIs abacavir, didanosine, emtricitabine, stavudine, tenofovir, and zidovudine, or with the gp41 fusion inhibitor enfuvirtide in cell culture. There was no antagonism of the cell culture combinations of tipranavir with either adefovir or ribavirin, used in the treatment of viral hepatitis.
Resistance
In cell culture:
HIV-1 isolates with a decreased susceptibility to tipranavir have been selected in cell culture and obtained from patients treated with APTIVUS/ritonavir (TPV/ritonavir). After 9 months of culture in TPV-containing medium, HIV-1 isolates with 87-fold reduced susceptibility to tipranavir were selected in cell culture; these contained 10 protease substitutions that developed in the following order: L33F, I84V, K45I, I13V, V32I, V82L, M36I, A71V, L10F, and I54V/T. Changes in the Gag polyprotein CA/P2 cleavage site were also observed following drug selection. Experiments with site-directed mutants of HIV-1 showed that the presence of 6 substitutions in the protease coding sequence (I13V, V32I, L33F, K45I, V82L, I84V) conferred >10-fold reduced susceptibility to tipranavir.
Clinical Studies of Treatment-Experienced Patients:
In controlled clinical trials 1182.12 and 1182.48, multiple protease inhibitor-resistant HIV-1 isolates from 59 treatment-experienced adult patients who received APTIVUS/ritonavir and experienced virologic rebound developed amino acid substitutions that were associated with resistance to tipranavir. The most common amino acid substitutions that developed on 500/200 mg APTIVUS/ritonavir in greater than 20% of APTIVUS/ritonavir virologic failure isolates were L33V/I/F, V82T, and I84V. Other substitutions that developed in 10 to 20% of APTIVUS/ritonavir virologic failure isolates included L10V/I/S, I13V, E35D/G/N, I47V, I54A/M/V, K55R, V82L, and L89V/M. Evolution at protease gag polyprotein cleavage sites was also observed. Among 28 pediatric patients in clinical trial 1182.14 who experienced virologic failure or non-response, the emergent protease amino acid codon substitutions were similar to those observed in adult virologic failure isolates.
In clinical trials 1182.12 and 1182.48 tipranavir resistance was detected at virologic rebound after an average of 38 weeks of APTIVUS/ritonavir treatment with a median 14-fold decrease in tipranavir susceptibility. Similarly, reduced tipranavir susceptibility was associated with emergent substitutions in pediatric patient isolates.
Cross-resistance
Cross-resistance among protease inhibitors has been observed. Tipranavir had <4-fold decreased susceptibility against 90% (94/105) of HIV-1 clinical isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, or saquinavir. Tipranavir-resistant viruses which emerged in cell culture from wild-type HIV-1 had decreased susceptibility to the protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir and ritonavir but remained sensitive to saquinavir.
Baseline Genotype and Virologic Outcome Analyses
Genotypic and/or phenotypic analysis of baseline virus may aid in determining tipranavir susceptibility before initiation of APTIVUS/ritonavir therapy. Several analyses were conducted to evaluate the impact of specific substitutions and combination of substitutions on virologic outcome. Both the type and number of baseline protease inhibitor substitutions as well as use of additional active agents (e.g., enfuvirtide) affected APTIVUS/ritonavir response rates in controlled clinical trials 1182.12 and 1182.48 through Week 48 of treatment.
Regression analyses of baseline and/or on-treatment HIV-1 genotypes from 860 treatment-experienced patients in Phase 2 and 3 trials demonstrated that amino acid substitutions at 16 codons in the HIV-1 protease coding sequence were associated with reduced virologic responses and/or reduced tipranavir susceptibility: L10V, I13V, K20M/R/V, L33F, E35G, M36I, K43T, M46L, I47V, I54A/M/V, Q58E, H69K, T74P, V82L/T, N83D or I84V.
As-treated analyses were also conducted to assess virologic outcome by the number of primary protease inhibitor substitutions present at baseline. Response rates were reduced if five or more protease inhibitor-associated substitutions were present at baseline and subjects did not receive concomitant new enfuvirtide with APTIVUS/ritonavir. See Table 9.
| Number of Baseline Primary PI Substitutionsa | APTIVUS/ritonavir N=578 | Comparator PI/ritonavir N=610 | ||
|---|---|---|---|---|
| No New Enfuvirtideb | + New Enfuvirtidec | No New Enfuvirtideb | + New Enfuvirtidec | |
| aPrimary PI substitutions include any amino acid substitution at positions 30, 32, 36, 46, 47, 48, 50, 53, 54, 82, 84, 88 and 90 | ||||
| bNo new enfuvirtide is defined as recycled or continued use of enfuvirtide or no use of enfuvirtide | ||||
| cNew enfuvirtide is defined as initiation of enfuvirtide for the first time | ||||
| Overall | 38% (180/470) | 69% (75/108) | 18% (92/524) | 26% (22/86) |
| 1 – 2 | 62% (24/39) | 60% (3/5) | 33% (14/43) | 0% (0/1) |
| 3 – 4 | 48% (96/202) | 71% (27/38) | 23% (45/193) | 38% (13/34) |
| 5+ | 26% (60/229) | 69% (45/65) | 11% (33/288) | 18% (9/51) |
The median change from baseline in plasma HIV-1 RNA at weeks 2, 4, 8, 16, 24 and 48 was evaluated by the number of baseline primary protease inhibitor resistance- associated substitutions (1-4 or ≥5) in subjects who received APTIVUS/ritonavir with or without new enfuvirtide. The following observations were made:
- Approximately 1.5 log10 decrease in HIV-1 RNA at early time points (Week 2) regardless of the number of baseline primary protease inhibitor resistance- associated substitutions (1-4 or 5+).
- Subjects with 5 or more primary protease inhibitor resistance-associated substitutions in their HIV-1 at baseline who received APTIVUS/ritonavir without new enfuvirtide (n=303) began to lose their antiviral response after Week 4.
- Early HIV-1 RNA decreases (1.5-2 log10) were sustained through Week 48 in subjects with 5 or more primary protease inhibitor resistance-associated substitutions at baseline who received new enfuvirtide with APTIVUS/ritonavir (n=74).
Baseline Phenotype and Virologic Outcome Analyses
APTIVUS/ritonavir response rates were also assessed by baseline tipranavir phenotype. Relationships between baseline phenotypic susceptibility to tipranavir, substitutions at protease amino acid codons 33, 82, 84 and 90, tipranavir resistance-associated substitutions, and response to APTIVUS/ritonavir therapy at Week-48 are summarized in Tables 10 and 11. These baseline phenotype groups are not meant to represent clinical susceptibility breakpoints for APTIVUS/ritonavir because the data are based on the select 1182.12 and 1182.48 patient population. The data are provided to give clinicians information on the likelihood of virologic success based on pre-treatment susceptibility to APTIVUS/ritonavir in protease inhibitor-experienced patients.
| Baseline Tipranavir Phenotype (Fold Change)a | Proportion of Respondersb with No New Enfuvirtidec Use N=211 | Proportion of Respondersb with New Enfuvirtided Use N=68 | Tipranavir Susceptibility |
|---|---|---|---|
| aChange in tipranavir EC50 value from wild-type reference | |||
| bConfirmed ≥1 log10 decrease at Week 48 | |||
| cNo new enfuvirtide is defined as recycled or continued use of enfuvirtide or no use of enfuvirtide | |||
| dNew enfuvirtide is defined as initiation of enfuvirtide for the first time | |||
| 0-3 | 48% (73/153) | 70% (33/47) | Susceptible |
| >3-10 | 21% (10/48) | 53% (8/15) | Decreased Susceptibility |
| >10 | 10% (1/10) | 50% (3/6) | Resistant |
| Baseline Tipranavir Phenotype (Fold Change)a | # of Baseline Protease Substitutions at 33, 82, 84, 90 | # of Baseline Tipranavir Resistance-Associated Substitutionsb | Tipranavir Susceptibilityc |
|---|---|---|---|
| aChange in tipranavir EC50 value from wild-type reference | |||
| bNumber of amino acid substitutions in HIV-1 protease among L10V, I13V, K20M/R/V, L33F, E35G, M36I, K43T, M46L, I47V, I54A/M/V, Q58E, H69K, T74P, V82L/T, N83D or I84V | |||
| cDefined by Week 48 response | |||
| 0-3 | 0-2 | 0-4 | Susceptible |
| >3-10 | 3 | 5-7 | Decreased Susceptibility |
| >10 | 4 | 8+ | Resistant |
Analyses of pediatric clinical trial 1182.14 also demonstrated that response to therapy was influenced by the number of baseline protease inhibitor substitutions present.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in mice and rats have been conducted with tipranavir. Mice were administered 30, 150 or 300 mg/kg/day tipranavir, 150/40 mg/kg/day tipranavir/ritonavir in combination, or 40 mg/kg/day ritonavir. The incidences of benign hepatocellular adenomas and combined adenomas/carcinomas were increased in females of all groups except the low dose of tipranavir. These tumors were also increased in male mice at the high-dose of tipranavir and the tipranavir/ritonavir combination group. Hepatocellular carcinoma incidence was increased in female mice given the high dose of tipranavir and both sexes receiving tipranavir/ritonavir. The combination of tipranavir and ritonavir caused an exposure-related increase in this same tumor type in both sexes. The clinical relevance of the carcinogenic findings in mice is unknown. Systemic exposures in mice (based on AUC or Cmax) at all dose levels tested were below those in humans receiving the recommended dose level. Rats were administered 30, 100 or 300 mg/kg/day tipranavir, 100/26.7 mg/kg/day tipranavir/ritonavir in combination, or 10 mg/kg/day ritonavir. No drug-related findings in male rats were observed. At the highest dose of tipranavir, an increased incidence of benign follicular cell adenomas of the thyroid gland was observed in female rats. Based on AUC measurements, exposure to tipranavir at this dose level in rats is approximately equivalent to exposure in humans at the recommended therapeutic dose. This finding is probably not relevant to humans, because thyroid follicular cell adenomas are considered a rodent-specific effect secondary to enzyme induction.
Tipranavir showed no evidence of mutagenicity or clastogenicity in a battery of five in vitro and in vivo tests including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, unscheduled DNA synthesis in rat hepatocytes, induction of gene mutation in Chinese hamster ovary cells, a chromosome aberration assay in human peripheral lymphocytes, and a micronucleus assay in mice.
Tipranavir had no effect on fertility or early embryonic development in rats at dose levels up to 1000 mg/kg/day, equivalent to a Cmax of 258 µM in females. Based on Cmax levels in these rats, as well as an exposure (AUC) of 1670 µM∙h in pregnant rats from another study, this exposure was approximately equivalent to the anticipated exposure in humans at the recommended dose level of 500/200 mg APTIVUS/ritonavir BID.
13.2 Animal Toxicology and/or Pharmacology
In preclinical studies in rats, tipranavir treatment induced dose-dependent changes in coagulation parameters (increased prothrombin time, increased activated partial thromboplastin time, and a decrease in some vitamin K dependent factors). In some rats, these changes led to bleeding in multiple organs and death. The co-administration of vitamin E in the form of TPGS (d-alpha-tocopherol polyethylene glycol 1000 succinate) with tipranavir resulted in a significant increase in effects on coagulation parameters, bleeding events, and death.
In preclinical studies of tipranavir in dogs, an effect on coagulation parameters was not seen. Co-administration of tipranavir and vitamin E has not been studied in dogs. Clinical evaluation of coagulation effects on HIV-1-infected patients demonstrated no tipranavir plus ritonavir effect and no effect of the vitamin E-containing oral solution on coagulation parameters [see Effects on Platelet Aggregation and Coagulation (5.4) ].
14. Clinical Studies
14.1 Adult Patients
The following clinical data is derived from analyses of 48-week data from ongoing studies measuring effects on plasma HIV-1 RNA levels and CD4+ cell counts. At present there are no results from controlled studies evaluating the effect of APTIVUS/ritonavir on clinical progression of HIV-1.
APTIVUS/ritonavir 500/200 mg BID + optimized background regimen (OBR) vs. Comparator Protease Inhibitor/ritonavir BID + OBR
The two clinical trials 1182.12 and 1182.48 (RESIST 1 and RESIST 2) are ongoing, randomized, controlled, open-label, multicenter studies in HIV-1 positive, triple antiretroviral class experienced patients. All patients were required to have previously received at least two protease inhibitor-based antiretroviral regimens and were failing a protease inhibitor-based regimen at the time of study entry with baseline HIV-1 RNA at least 1000 copies/mL and any CD4+ cell count. At least one primary protease gene mutation from among 30N, 46I, 46L, 48V, 50V, 82A, 82F, 82L, 82T, 84V or 90M had to be present at baseline, with not more than two mutations at codons 33, 82, 84 or 90.
These studies evaluated treatment response at 48 weeks in a total of 1483 patients receiving either APTIVUS co-administered with 200 mg of ritonavir plus OBR versus a control group receiving a ritonavir-boosted protease inhibitor (lopinavir, amprenavir, saquinavir or indinavir) plus OBR. Prior to randomization, OBR was individually defined for each patient based on genotypic resistance testing and patient history. The investigator had to declare OBR, comparator protease inhibitor, and use of new enfuvirtide prior to randomization. Randomization was stratified by choice of comparator protease inhibitor and use of new enfuvirtide.
After Week 8, patients in the control group who met the protocol defined criteria of initial lack of virologic response or confirmed virologic failure had the option of discontinuing treatment and switching to APTIVUS/ritonavir in a separate roll-over study.
Demographics and baseline characteristics were balanced between the APTIVUS/ritonavir arm and control arm. In both studies combined, the 1483 patients had a median age of 43 years (range 17-80), and were 86.3% male, 75.6% white, 12.9% black, and 0.9% Asian. The median baseline plasma HIV-1 RNA for both treatment groups was 4.8 (range 2.0 to 6.8) log10 copies/mL and median baseline CD4+ cell count was 162 (range 1 to 1894) cells/mm3. Overall, 38.4% of patients had a baseline HIV-1 RNA of >100,000 copies/mL, 58.6% had a baseline CD4+ cell count ≤200 cells/mm3, and 57.8% had experienced an AIDS defining Class C event at baseline.
Patients had prior exposure to a median of 6 NRTIs, 1 NNRTI, and 4 PIs. A total of 10.1% of patients had previously used enfuvirtide. In baseline patient samples (n=454), 97% of the HIV-1 isolates were resistant to at least one protease inhibitor, 95% of the isolates were resistant to at least one NRTI, and >75% of the isolates were resistant to at least one NNRTI.
The individually pre-selected protease inhibitor based on genotypic testing and the patient's medical history was lopinavir in 48.7%, amprenavir in 26.4%, saquinavir in 21.8% and indinavir in 3.1% of patients. A total of 85.1% were possibly resistant or resistant to the pre-selected comparator protease inhibitors. Approximately 21% of patients used enfuvirtide during the study of which 16.6% in the APTIVUS/ritonavir arm and 13.2% in the comparator/ritonavir arm represented first time use of enfuvirtide (new enfuvirtide).
Treatment response and efficacy outcomes of randomized treatment through Week 48 of studies 1182.12 and 1182.48 are shown in Table 12.
| Outcome | APTIVUS/ritonavir (500/200 mg BID) + OBR (N=746) | Comparator Protease Inhibitor*/ritonavir + OBR (N=737) | ||
|---|---|---|---|---|
| *Comparator protease inhibitors were lopinavir, amprenavir, saquinavir or indinavir and 85.1% of patients were possibly resistant or resistant to the chosen protease inhibitors. | ||||
| aPatients achieved and maintained a confirmed ≥1 log10 HIV-1 RNA drop from baseline through Week 48 without prior evidence of treatment failure. | ||||
| bPatients did not achieve a 0.5 log10 HIV-1 RNA drop from baseline and did not have viral load <100,000 copies/mL by Week 8. | ||||
| cDeath only counted if it was the reason for treatment failure. | ||||
| dIncludes patients who were lost to-follow-up, withdrawn consent, non-adherent, protocol violations, added/changed background antiretroviral drugs for reasons other than tolerability or toxicity, or discontinued while suppressed. | ||||
| Virologic Respondersa (confirmed at least 1 log10 HIV-1 RNA below baseline) | 33.8% | 14.9% | ||
| Virologic failures | 55.1% | 77.3% | ||
| Initial lack of virologic response by Week 8b | 33.0% | 57.9% | ||
| Rebound | 18.9% | 16.4% | ||
| Never suppressed | 3.2% | 3.0% | ||
| Deathc or discontinued due to adverse events | 5.9% | 1.9% | ||
| Death | 0.5% | 0.3% | ||
| Discontinued due to adverse events | 5.4% | 1.6% | ||
| Discontinued due to other reasonsd | 5.2% | 5.8% | ||
Through 48 weeks of treatment, the proportion of patients in the APTIVUS/ritonavir arm compared to the comparator PI/ritonavir arm with HIV-1 RNA <400 copies/mL was 30.3% and 13.6% respectively, and with HIV-1 RNA <50 copies/mL was 22.7% and 10.2% respectively. Among all randomized and treated patients, the median change from baseline in HIV-1 RNA at the last measurement up to Week 48 was -0.64 log10 copies/mL in patients receiving APTIVUS/ritonavir versus -0.22 log10 copies/mL in the comparator PI/ritonavir arm.
Among all randomized and treated patients, the median change from baseline in CD4+ cell count at the last measurement up to Week 48 was +23 cells/mm3 in patients receiving APTIVUS/ritonavir (N=740) versus +4 cells/mm3 in the comparator PI/ritonavir (N=727) arm.
Patients in the APTIVUS/ritonavir arm achieved a significantly better virologic outcome when APTIVUS/ritonavir was combined with enfuvirtide. Among patients with new enfuvirtide use, the proportion of patients in the APTIVUS/ritonavir arm compared to the comparator PI/ritonavir arm with HIV-1 RNA <400 copies/mL was 52.4% and 19.6% respectively, and with HIV-1 RNA <50 copies/mL was 37.3% and 14.4% respectively [see Clinical Pharmacology (12.2) and Microbiology (12.4) ]. The median change from baseline in CD4+ cell count at the last measurement up to Week 48 was +89 cells/mm3 in patients receiving APTIVUS/ritonavir in combination with newly introduced enfuvirtide (N=124) and +18 cells/mm3 in the comparator PI/ritonavir (N=96) arm.
14.2 Pediatric Patients
The pharmacokinetic profile, safety and activity of APTIVUS/ritonavir was evaluated in a randomized, open-label, multicenter study. This study enrolled HIV-1 infected, treatment-experienced pediatric patients (with the exception of 3 treatment-naïve patients), with baseline HIV-1 RNA of at least 1500 copies/mL. The age ranged from 2 through 18 years and patients were stratified by age (2 to <6 years, 6 to <12 years and 12 to 18 years). One hundred and ten (110) patients were randomized to receive one of two APTIVUS/ritonavir dose regimens: 375 mg/m2/150 mg/m2 dose (N=55) or 290 mg/m2/115 mg/m2 dose (N=55), plus background therapy of at least two non-protease inhibitor antiretroviral drugs, optimized using baseline genotypic resistance testing. All patients initially received APTIVUS oral solution. Pediatric patients who were 12 years or older and received the maximum dose of 500/200 mg BID could subsequently change to APTIVUS capsules at day 28 [see Adverse Reactions (6.2), Use in Specific Populations (8.4), Clinical Pharmacology (12.3), and Microbiology (12.4) ].
Demographics and baseline characteristics were balanced between the APTIVUS/ritonavir dose groups. The 110 randomized pediatric patients had a median age of 11.7 years (range 2 to 18), and were 57.2% male, 68.1% white, 30% black, and 1.8% Asian. The median baseline plasma HIV-1 RNA was 4.7 (range 3.0 to 6.8) log10 copies/mL and median baseline CD4+ cell count was 379 (range 2 to 2578) cells/mm3. Overall, 37.4% of patients had a baseline HIV-1 RNA of >100,000 copies/mL; 28.7% had a baseline CD4+ cell count ≤200 cells/mm3, and 48% had experienced a prior AIDS defining Class C event at baseline. Patients had prior exposure to a median of 4 NRTIs, 1 NNRTI, and 2 PIs.
Eighty three (75%) completed the 48 week period while 25% discontinued prematurely. Of the patients who discontinued prematurely, 9 (8%) discontinued due to virologic failure, and 9 (8%) discontinued due to adverse reactions.
At 48 weeks, 40% of patients had viral load <400 copies/mL. The proportion of patients with viral load <400 copies/mL tended to be greater (70%) in the youngest group of patients, who had less baseline viral resistance, compared to the older groups (37% and 31%). The HIV-1 RNA results are presented in Table 13.
| APTIVUS/ritonavir Dose Regimen | 2 to <6 years (N=20) | 6 to <12 years (N=38) | 12 to 18 years (N=52) |
|---|---|---|---|
| * The number of baseline tipranavir resistance-associated substitutions were fewer in the 2 to <6 year old patients than the 6 to 18 year old patients enrolled in study 1182.14 | |||
| 375 mg/m2/150 mg/m2 | n=10 70% (42%) | n=19 50% (39%) | n=26 33% (30%) |
| 290 mg/m2/115 mg/m2 | n=10 70% (54%) | n=19 37% (32%) | n=26 31% (23%) |
The dose selection for all age groups was based on the following:
- A greater proportion of patients receiving APTIVUS/ritonavir 375 mg/m2/150 mg/m2 compared to 290 mg/m2/115 mg/m2 achieved HIV-1 RNA <400 and <50 copies/mL.
- A greater proportion of patients 6 to 18 years of age with multiple baseline protease inhibitor resistance-associated substitutions receiving APTIVUS/ritonavir 375 mg/m2/150 mg/m2 achieved HIV-1 RNA <400 copies/mL at 48 weeks compared to patients receiving APTIVUS/ritonavir 290 mg/m2/115 mg/m2.
- No clinically significant increase in adverse event rates observed with 375 mg/m2/150 mg/m2 compared to 290 mg/m2/115 mg/m2.
- Overall, 6 (5%) patients ages 6 to 18 had AIDS defining illness during the treatment period and all received the 290 mg/m2/115 mg/m2 dose.
The guidance for possible dose reduction for patients who develop intolerance or toxicity and cannot continue with APTIVUS/ritonavir 14 mg/kg/6 mg/kg (or 375 mg/m2/150 mg/m2) is based on the following:
- The 290 mg/m2/115 mg/m2 twice daily regimen provided tipranavir plasma concentrations similar to those obtained in adults receiving 500/200 mg twice daily. The 375 mg/m2/150 mg/m2 twice daily regimen provided tipranavir plasma concentrations 37% higher than those obtained in adults receiving 500/200 mg twice daily.
- The observed response rates for APTIVUS/ritonavir dose of 290 mg/m2/115 mg/m2 as shown in Table 13.
Dose reduction is not appropriate for patients whose virus is resistant to more than one protease inhibitor.
When body surface area (BSA) dosing is converted to mg/kg dosing, the APTIVUS/ritonavir 375 mg/m2/150 mg/m2 twice daily regimen is similar to 14 mg/kg/6 mg/kg and APTIVUS/ritonavir 290 mg/m2/115 mg/m2 twice daily regimen is similar to 12 mg/kg/5 mg/kg twice daily [see Dosage and Administration (2.2) ].
16. How Supplied/Storage and Handling
APTIVUS capsules 250 mg are pink, oblong soft gelatin capsules imprinted in black with "TPV 250". They are packaged in HDPE unit-of-use bottles with a child resistant closure and 120 capsules. (NDC 0597-0003-02).
APTIVUS oral solution is a clear yellow viscous buttermint-butter toffee flavored liquid containing 100 mg tipranavir in each mL. The solution is supplied in a unit-of-use amber glass bottle providing 95 mL of solution with a child resistant closure. A 5 mL plastic oral dispensing syringe is also provided. (NDC 0597-0002-01).
Storage
- APTIVUS capsules should be stored in a refrigerator 2°-8°C (36°-46°F) prior to opening the bottle. After opening the bottle, the capsules may be stored at 20°C-25°C (68°F-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature] and must be used within 60 days after first opening of the bottle.
- APTIVUS oral solution should be stored at 15°-25°C (59°-77°F). Do not refrigerate or freeze. The solution must be used within 60 days after first opening of the bottle.
Store in a safe place out of the reach of children.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
• Hepatic Impairment and Toxicity
Inform patients that APTIVUS co-administered with 200 mg of ritonavir, has been associated with severe liver disease, including some deaths. Patients with signs or symptoms of clinical hepatitis should discontinue APTIVUS/ritonavir treatment and seek medical evaluation. Symptoms of hepatitis include fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. Extra vigilance is needed for patients with chronic hepatitis B or C co-infection, as these patients have an increased risk of developing hepatotoxicity.
Liver function tests should be performed prior to initiating therapy with APTIVUS and 200 mg of ritonavir, and frequently throughout the duration of treatment. Patients with chronic hepatitis B or C co-infection or elevations in liver enzymes prior to treatment are at increased risk (approximately 2-fold) for developing further liver enzyme elevations or severe liver disease. Caution should be exercised when administering APTIVUS/ritonavir to patients with liver enzyme abnormalities or history of chronic liver disease. Increased liver function testing is warranted in these patients. APTIVUS should not be given to patients with moderate to severe hepatic impairment.
• Intracranial Hemorrhage
Inform patients that APTIVUS co-administered with 200 mg of ritonavir has been associated with reports of both fatal and non-fatal intracranial hemorrhage. Patients should report any unusual or unexplained bleeding to their physician.
• Drug Interactions
APTIVUS may interact with some drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or non-prescription medications or herbal products, particularly St. John's wort.
• Use of Vitamin E
Advise patients taking APTIVUS oral solution not to take supplemental vitamin E greater than a standard multivitamin as APTIVUS oral solution contains 116 IU/mL of vitamin E and when taken at the recommended maximum dose of 500 mg/200 mg tipranavir/ritonavir BID, results in a daily dose of 1160 IU. This intake is higher than the Reference Daily Intake (adults 30 IU, pediatrics approximately 10 IU).
• Rash
Rash, including flat or raised rashes or sensitivity to the sun, have been reported in approximately 10% of subjects receiving APTIVUS. Some patients who developed rash also had one or more of the following symptoms: joint pain or stiffness, throat tightness, generalized itching, muscle aches, fever, redness, blisters, or peeling of the skin. Women taking birth control pills may get a skin rash. Tell patients to discontinue use of APTIVUS and call their physician right away if any of these symptoms develop.
• Contraceptives
Instruct women receiving estrogen-based hormonal contraceptives that additional or alternative contraceptive measures should be used during therapy with APTIVUS. There may be an increased risk of rash when APTIVUS is given with hormonal contraceptives [see Use in Specific Populations (8.3) ].
• Fat Redistribution
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
• Administration
Inform patients that APTIVUS must be co-administered with ritonavir to ensure its therapeutic effect. Failure to correctly co-administer APTIVUS with ritonavir will result in reduced plasma levels of tipranavir that may be insufficient to achieve the desired antiviral effect.
- APTIVUS co-administered with ritonavir capsules or solution can be taken with or without meals
- APTIVUS co-administered with ritonavir tablets must only be taken with meals
Instruct patients to swallow APTIVUS capsules whole. They must not be opened or chewed.
Tell patients that sustained decreases in plasma HIV-1 RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain under the care of a physician while using APTIVUS. Advise patients to take APTIVUS and other concomitant antiretroviral therapy every day as prescribed. APTIVUS, co-administered with ritonavir, must be given in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting with their healthcare professional. If a dose of APTIVUS is missed, patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped the patient should not double the next dose.
• Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to APTIVUS during pregnancy [see Use in Specific Populations (8.1) ].
• Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see Use in Specific Populations (8.2) ].
Spl Unclassified Section
Distributed by:Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA
Address medical inquiries to: (800) 542-6257 or (800) 459-9906 TTY.
APTIVUS® is a registered trademark used under license from Boehringer Ingelheim International GmbH.
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
Copyright © 2020 Boehringer Ingelheim International GmbHALL RIGHTS RESERVED
OT2000VF232020
Patient Package Insert
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 06/2020 | ||
| PATIENT INFORMATION | |||
| APTIVUS® (AP-tih-vus) (tipranavir) capsules | APTIVUS® (AP-tih-vus) (tipranavir) oral solution | ||
| What is the most important information I should know about APTIVUS? APTIVUS can cause serious side effects, including:
| |||
|
| ||
| |||
| What is APTIVUS? APTIVUS is a prescription medicine used with ritonavir and other anti-HIV-1 medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection in people who:
It is not known if APTIVUS is safe and effective in children under 2 years of age. | |||
Do not take APTIVUS if you:
| |||
Before taking APTIVUS, tell your healthcare provider about all of your medical conditions, including if you:
| |||
How should I take APTIVUS?
| |||
What are the possible side effects of APTIVUS? APTIVUS may cause serious side effects, including:
| |||
|
| ||
| |||
|
| ||
| The most common side effects of APTIVUS in children were the same as those seen in adults. Rash was seen more frequently in children than in adults. These are not all of the possible side effects of APTIVUS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
How should I store APTIVUS?
| |||
| General information about the safe and effective use of APTIVUS. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use APTIVUS for a condition for which it was not prescribed. Do not give APTIVUS to other people, even if they have the same condition you have. It may harm them. You can ask your pharmacist or healthcare professional for information about APTIVUS that is written for health professionals. | |||
| What are the ingredients in APTIVUS? Capsules: Active Ingredient: tipranavir Major Inactive Ingredients: dehydrated alcohol, polyoxyl 35 castor oil, propylene glycol, mono/diglycerides of caprylic/capric acid, and gelatin. Oral Solution: Active Ingredient: tipranavir Major Inactive Ingredients: polyethylene glycol 400, vitamin E polyethylene glycol succinate, purified water, and propylene glycol. Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA APTIVUS® is a registered trademark used under license from Boehringer Ingelheim International GmbH. Copyright © 2020 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED OT2000VF232020 For current prescribing information, scan the code below or for additional information, you may also call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257, or 1-800-459-9906 TTY.
| |||
Package Label. Principal Display Panel
NDC 0597-0003-02
Aptivus®(tipranavir)Capsules 250 mg
120 capsules Unit of Use Container
Each capsule contains 250 mg tipranavir and 7% w/w dehydrated alcohol (0.1 g per capsule).
ALERTFind out about medicines that shouldnot be taken with Aptivus
Note to Pharmacist: Do not cover ALERT box with Pharmacy label
Boehringer Ingelheim
Rx only

 Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy
Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy Darunavir-Cobicistat-Tenofovir alafenamide-Emtricitabine Symtuza
Darunavir-Cobicistat-Tenofovir alafenamide-Emtricitabine Symtuza Dolutegravir-Abacavir-Lamivudine Triumeq
Dolutegravir-Abacavir-Lamivudine Triumeq Dolutegravir-Lamivudine Dovato
Dolutegravir-Lamivudine Dovato Dolutegravir-Rilpivirine Juluca
Dolutegravir-Rilpivirine Juluca Doravirine-Tenofovir DF-Lamivudine Delstrigo
Doravirine-Tenofovir DF-Lamivudine Delstrigo Efavirenz-Tenofovir DF-Emtricitabine Atripla
Efavirenz-Tenofovir DF-Emtricitabine Atripla Elvitegravir-Cobicistat-Tenofovir alafenamide-Emtricitabine Genvoya
Elvitegravir-Cobicistat-Tenofovir alafenamide-Emtricitabine Genvoya Elvitegravir-Cobicistat-Tenofovir DF-Emtricitabine Stribild
Elvitegravir-Cobicistat-Tenofovir DF-Emtricitabine Stribild Rilpivirine-Tenofovir alafenamide-Emtricitabine Odefsey
Rilpivirine-Tenofovir alafenamide-Emtricitabine Odefsey Rilpivirine-Tenofovir DF-Emtricitabine Complera
Rilpivirine-Tenofovir DF-Emtricitabine Complera Enfuvirtide Fuzeon
Enfuvirtide Fuzeon Fostemsavir Rukobia
Fostemsavir Rukobia Ibalizumab Trogarzo
Ibalizumab Trogarzo Maraviroc Selzentry
Maraviroc Selzentry Dolutegravir Tivicay
Dolutegravir Tivicay Raltegravir Isentress
Raltegravir Isentress Abacavir Ziagen
Abacavir Ziagen Abacavir-Lamivudine Epzicom
Abacavir-Lamivudine Epzicom Abacavir-Lamivudine-Zidovudine Trizivir
Abacavir-Lamivudine-Zidovudine Trizivir Didanosine Videx
Didanosine Videx Emtricitabine Emtriva
Emtricitabine Emtriva Lamivudine Epivir
Lamivudine Epivir Stavudine Zerit
Stavudine Zerit Tenofovir alafenamide-Emtricitabine Descovy
Tenofovir alafenamide-Emtricitabine Descovy Tenofovir DF Viread
Tenofovir DF Viread Tenofovir DF-Emtricitabine Truvada and Multiple Generics
Tenofovir DF-Emtricitabine Truvada and Multiple Generics Zidovudine Retrovir
Zidovudine Retrovir Zidovudine-Lamivudine Combivir
Zidovudine-Lamivudine Combivir Doravirine Pifeltro
Doravirine Pifeltro Efavirenz Sustiva
Efavirenz Sustiva Etravirine Intelence
Etravirine Intelence Nevirapine Viramune
Nevirapine Viramune Rilpivirine Edurant
Rilpivirine Edurant Atazanavir Reyataz
Atazanavir Reyataz Atazanavir-Cobicistat Evotaz
Atazanavir-Cobicistat Evotaz Darunavir Prezista
Darunavir Prezista Darunavir-Cobicistat Prezcobix
Darunavir-Cobicistat Prezcobix Fosamprenavir Lexiva
Fosamprenavir Lexiva Indinavir Crixivan
Indinavir Crixivan Lopinavir-Ritonavir Kaletra
Lopinavir-Ritonavir Kaletra Nelfinavir Viracept
Nelfinavir Viracept Saquinavir Invirase
Saquinavir Invirase Tipranavir Aptivus
Tipranavir Aptivus Cobicistat Tybost
Cobicistat Tybost Ritonavir Norvir
Ritonavir Norvir