In the early 1990s, HIV occupational postexposure prophylaxis (PEP) was recognized as a safe and effective intervention to prevent the acquisition of HIV for health care workers exposed to HIV-contaminated blood or body fluids.[1] In conjunction with these findings, the Centers for Disease Control and Prevention (CDC) issued several publications in the 1990’s that provided specific recommendations and guidance for HIV occupational PEP.[2] In 2005, the CDC extended these recommendations to the nonoccupational setting, with the first publication of HIV nonoccupational PEP guidelines.[3] The initial 2005 HIV nonoccupational PEP guidelines were subsequently updated in 2016 and again, very recently, in 2025.[4,5] The HIV nonoccupational PEP guidelines& address exposures to HIV that occur through sexual contact and/or through injection drug use, as well as other less common routes.[4] Clinicians evaluating individuals for HIV nonoccupational PEP should provide general HIV prevention counseling and recognize that many of these individuals may be excellent candidates to transition to HIV preexposure prophylaxis (PrEP) at the time they complete the 28-day regimen for HIV nonoccupational PEP.[4,6]
- Module 5 Overview
Prevention of HIV - 0%Lesson 1
Preventing Perinatal HIV TransmissionActivities- 0%Lesson 2
Preventing HIV Transmission in Persons with HIVActivities- 0%Lesson 3
Occupational Postexposure ProphylaxisLesson 4. Nonoccupational Postexposure Prophylaxis
Learning Objective Performance Indicators
- Describe indications for the use of HIV nonoccupational postexposure prophylaxis (PEP)
- Summarize appropriate antiretroviral therapy regimens and duration of therapy for HIV nonoccupational PEP
- List recommended clinical and laboratory monitoring following nonoccupational exposure to HIV
- Identify appropriate candidates for transition from HIV nonoccupational PEP to HIV preexposure prophylaxis (PrEP)
- Discuss indications for obtaining expert consultation for HIV nonoccupational PEP
Last Updated: June 24th, 2025Authors:David H. Spach, MD,David H. Spach, MD
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneAley G. Kalapila, MD, PhDAley G. Kalapila, MD, PhD
Professor of Medicine
Division of Infectious Diseases
Emory University School of Medicine
Grady Health SystemDisclosures: NoneReviewer:Jehan Z. Budak, MDJehan Z. Budak, MD
Associate Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneTable of Contents- Nonoccupational Postexposure Prophylaxis
- Introduction and Background
- Rationale for Providing HIV Nonoccupational PEP
- Evaluation for HIV Nonoccupational PEP
- Indications for Initiating HIV Nonoccupational PEP
- Indications for HIV Nonoccupational PEP After Sexual Exposure to HIV
- Indications for HIV Nonoccupational PEP After Injection Drug Exposure
- Indications for HIV Nonoccupational PEP After Possible Other Exposures
Recommendation if Source Person Has Sustained Viral Suppression
Recommendation if Person Exposed to HIV is Taking HIV PrEP
- Recommended Regimens for HIV Nonoccupational PEP
- Time to Initiation of HIV Nonoccupational PEP
- Duration of Therapy
- HIV Nonoccupational PEP Regimens for Adults and Adolescents
- HIV Nonoccupational PEP Regimens in Pregnant Women
- HIV Nonoccupational PEP Regimens in Women who are Breastfeeding
- HIV Nonoccupational PEP Regimens for Children and Adolescents
- HIV Nonoccupational PEP Regimens in Patients with Renal Dysfunction or Hepatic Insufficiency
- Expert Consultation for HIV Nonoccupational PEP
- Baseline and Follow-Up Laboratory Testing
- HIV Nonoccupational PEP After Sexual Assault
- Initial Medication Prescription and Follow-up after Evaluation
- Transitioning from HIV Nonoccupational PEP to HIV PrEP
- Nonoccupational PEP for Infectious Pathogens Other Than HIV
- Summary Points
- Citations
- Additional References
- Figures
- Tables
Introduction and Background
Rationale for Providing HIV Nonoccupational PEP
Due to ethical and logistical reasons, it is highly unlikely that a prospective, randomized, placebo-controlled trial to evaluate HIV nonoccupational PEP in humans will ever take place. In addition, HIV nonoccupational PEP studies in humans are challenging because participants may have multiple exposures over the surveillance-testing period, making it difficult to discern the true benefit of HIV nonoccupational PEP for a single exposure event.[7] Thus, the rationale for providing HIV nonoccupational PEP is based on extrapolation from the use of HIV PEP in other settings, animal studies, retrospective reviews, observational HIV nonoccupational PEP reports, and expert opinion.[4]
Occupational and Perinatal HIV PEP Data
In 1997, investigators reported findings from a case-control study involving health care workers who sustained needlestick injuries from source individuals with HIV; this study demonstrated HIV occupational PEP with oral zidovudine, taken within 4 hours by most of the participants, reduced the risk of HIV seroconversion by 81%.[8] In addition, several important perinatal transmission trials involving mothers with HIV have established a reduction in HIV transmission when using HIV PEP given to the mother during labor and/or to the baby following birth.[9,10,11,12]
Animal PEP Studies
In an early animal HIV PEP study, investigators inoculated macaques intravenously with simian immunodeficiency virus (SIV) and showed that tenofovir PEP reduced the rate of SIV seroconversion, with the greatest reduction in transmission achieved when prophylaxis was initiated as early as possible and continued for 28 days (Figure 1).[13] A later study showed that tenofovir-based HIV PEP is also effective in preventing HIV acquisition after intravaginal inoculation of female macaques with HIV-2: tenofovir prevented seroconversion in all 8 of the female macaques exposed to HIV-2 when initiated within 12 to 36 hours.[14] A systematic review and meta-analysis of HIV PEP using pooled data of nonhuman primates across 18 studies (mostly involving intravenous inoculation with HIV) further substantiated the efficacy of HIV PEP when initiated as soon as possible after HIV exposure.[15]
Rationale for HIV Nonoccupational PEP in Persons Who Inject Drugs
New HIV infections among persons who inject drugs can potentially result from injection drug use or from sexual activity.[16] Certain circumstances could arise whereby a person who injects drugs and normally uses safe injection practices has an HIV risk exposure. The use of HIV nonoccupational PEP after an at-risk injection drug use exposure may have different efficacy compared with use after a sexual exposure, since the route and HIV inoculum differ in these two situations.[17] It is important that programs, including syringe services programs, that work with people who inject drugs are aware of local resources where their clients can receive HIV nonoccupational PEP if needed. In addition, these programs may consider directly providing services for HIV nonoccupational PEP if they have the capacity and expertise.
HIV Nonoccupational PEP Data in Humans
Although human studies on HIV nonoccupational PEP are observational in nature and limited in sample size, available data involving men who have sex with men (MSM) suggest HIV nonoccupational PEP reduces HIV transmission.[18,19] In addition, a feasibility study in San Francisco demonstrated that medical providers could appropriately identify and provide recommended HIV nonoccupational PEP to persons exposed to HIV via sexual contact or through injecting drugs.[20] In a separate San Francisco study, investigators reported HIV seroconversion among 7 of 702 (1%) persons who received HIV nonoccupational PEP, but only 3 of the seroconversions likely represented true “failure” of HIV nonoccupational PEP.[7] Available data from other reports of HIV transmission in persons who received HIV nonoccupational PEP suggest that most HIV transmissions resulted from poor medication adherence or from exposures to HIV that occurred after completing the HIV nonoccupational PEP regimen.[21,22,23,24] In addition, one failure occurred in a 40-year-old woman in France who started HIV nonoccupational PEP more than 72 hours after a sexual exposure.[25] The following summarizes several major studies involving the preferred and alternative regimens recommended for HIV nonoccupational PEP.
- Bictegravir-tenofovir alafenamide-emtricitabine: Two studies have been published using bictegravir-tenofovir alafenamide-emtricitabine for HIV nonoccupational PEP.[26,27] In an open-label study, 52 individuals who accessed nonoccupational HIV PEP services at an urban health center were enrolled to receive coformulated bictegravir-tenofovir alafenamide-emtricitabine 1 tablet orally per day for 28 days.[27] For the participants, this regimen was well tolerated, safe, and highly acceptable; in this study, there were no HIV seroconversions related to the exposure.[27] In a prospective, open-label study conducted in China, 112 participants received a 28-day course of bictegravir-tenofovir alafenamide-emtricitabine for HIV nonoccupational PEP.[26] The HIV nonoccupational PEP regimen was safe, well tolerated, and no participants acquired HIV (when followed out to 24 weeks).[26]
- Dolutegravir plus tenofovir DF-emtricitabine: Three studies have been published that involved dolutegravir plus tenofovir DF-emtricitabine for HIV nonoccupational PEP, including one open-label single-arm trial, one retrospective study, and one cohort study.[28,29,30] In an open-label, single-arm study from Sydney, Australia, 100 men who have sex with men (MSM) received dolutegravir plus tenofovir DF-emtricitabine for 28 days for HIV nonoccupational PEP.[28] The regimen was well tolerated, completion rates were high (90%), and no HIV seroconversions occurred; elevations in alanine aminotransferase occurred in 22% of the participants, but none developed clinical hepatitis.[28] In a French retrospective analysis involving 19,240 nonoccupational exposures to HIV, 12 different HIV nonoccupational PEP regimens were prescribed, including 704 exposures where dolutegravir plus tenofovir DF-emtricitabine was used.[29] Among those taking dolutegravir plus tenofovir DF-emtricitabine, 87.2% completed the course, and there were no new HIV infections.[29] In a cohort study in China, one of the arms included 100 persons who took dolutegravir plus tenofovir DF-emtricitabine, and there were no seroconversions among those who took this regimen.[30]
- Darunavir (boosted with ritonavir): In an observational study, 51 individuals took darunavir boosted with ritonavir plus tenofovir DF-emtricitabine for HIV nonoccupational PEP, and 6 (12%) of the individuals discontinued due to medication side effects.[31] In a 2015 systematic review of 25 HIV nonoccupational PEP studies, the completion rate with darunavir and ritonavir plus tenofovir DF-emtricitabine was 94%, which was significantly higher than the 71% completion rate with lopinavir-ritonavir plus tenofovir DF-emtricitabine.[32] There are inadequate data regarding failure rates with darunavir and ritonavir-based regimens.
Evaluation for HIV Nonoccupational PEP
Multiple factors influence the risk of HIV transmission in nonoccupational exposures to HIV. The initial evaluation of a person seeking care after a potential nonoccupational exposure to HIV, including a sexual or injection drug exposure, requires promptly gathering information to determine whether HIV nonoccupational PEP is indicated.[4] This initial evaluation, as recommended in the 2025 HIV nPEP Recommendations, should address the following: (1) details regarding the timing of when the exposure occurred, (2) the type of exposure(s) involved, (3) information, if known, about the HIV status of the person potentially exposed to HIV, (4) information related to the source person’s HIV status, and (5) any available information related to the antiretroviral therapy taken by the source patient, including information on whether they have sustained suppression of HIV RNA levels.[4] The following summarizes more detailed information about determining whether HIV nonoccupational PEP is indicated.[4]
- Timing of Exposure: It is important to determine the timing of exposure in persons seeking HIV nonoccupational PEP. Available data suggest that HIV nonoccupational PEP may not be effective if initiated more than 72 hours after the exposure. Furthermore, it is important to determine whether multiple exposures have occurred; if multiple exposures have occurred, there is still a need to assess whether any of the exposures have occurred within the previous 72 hours. If the person reports frequent, recurrent HIV exposures, then repeated courses of HIV nonoccupational PEP are not an optimal long-term HIV prevention strategy; these individuals should ideally receive HIV PrEP.[4]
- Type of Exposure: For sexual exposures involving vaginal or anal sex, it is important to determine what type of sex occurred (anal intercourse, and/or vaginal intercourse, and/or oral genital sex). In addition, it is important to know whether a condom was used, and if so, whether it was used during the entire sexual exposure. For exposures involving injection drug use, information should be obtained regarding the sharing of needles (or other drug injection equipment) with another person or persons.
- Information on HIV Status of Person Potentially Exposed to HIV: As part of the initial evaluation, it is important to determine the baseline HIV status of the person seeking medical care for the exposure. In the setting of a nonoccupational exposure to HIV, determining the HIV status of the exposed person should not delay the initiation of HIV nonoccupational PEP. If HIV nonoccupational PEP is started and the person’s baseline test is positive for HIV (indicating they already have HIV), then additional evaluation, including a baseline HIV RNA level and HIV drug resistance testing, should promptly occur, and the person with HIV should receive long-term continuous antiretroviral therapy, not a 28-day course of HIV nonoccupational PEP.
- Information Related to Source Person’s HIV Status: As part of the initial exposure evaluation, it is also important to determine whether the source person is known to have HIV. If the status of the source is unknown, assess their risk factors for HIV, if possible. If exposures have occurred with multiple people who may have HIV, then every effort should be made to get information on the HIV status of all source persons involved. Often, the HIV status of the source person is not known (and not attainable). If the HIV status of the source person is not known, then initiating HIV nonoccupational PEP should occur on a case-by-case basis, ideally in consultation with an expert.[4] In this situation, if the exposure type warrants HIV nonoccupational PEP and the person has sought care within 72 hours, most experts would recommend initiating and completing a 28-day course of HIV nonoccupational PEP.
- Source Person’s Antiretroviral Treatment Information: If a source person is known to have HIV and takes antiretroviral medications, the medical provider should determine what medication the source takes, the most recent HIV RNA levels (ideally a result within the prior 2 months), and if the source person has drug-resistant HIV. The risk of HIV transmission is higher if the source person has elevated HIV RNA levels.[33] In contrast, multiple studies consistently report that persons with HIV who take antiretroviral therapy and maintain plasma HIV RNA levels of less than 200 copies/mL do not transmit HIV sexually to their partners—this concept is referred to as Undetectable = Untransmittable (U=U).[34,35] Similar studies have not been published related to HIV RNA levels and HIV transmission through injection drug use.[35]
Determination of Risk Related to Exposure
The other key element of the initial HIV nonoccupational PEP evaluation is determining the relative risk of HIV transmission based on the exposure.[5,17] Administering HIV nonoccupational PEP should occur only in the setting of substantial risk for HIV transmission, defined as contact involving an area of the body known to be associated with HIV acquisition (vagina, rectum, eye, mouth, or other mucous membranes, non-intact skin, or percutaneous needlestick injuries) with an infectious body fluid (e.g., blood, semen, vaginal secretions, rectal secretions, breast milk, or any other body fluid visibly contaminated with blood). The risk of HIV transmission associated with nonoccupational exposures varies considerably by the type of sexual exposure, with receptive anal intercourse conveying the highest sexual risk.[5,17,36,37,38] The following table summarizes the risk of HIV acquisition based on the type of exposure.[4](Table 1)
Risk Estimates Specific to Sexual Exposures
Mucosal disruption in either the source person or the exposed person (as might occur with traumatic intercourse, including sexual assault, or in the presence of ulcerative genital disease) increases the risk of sexual HIV transmission; correct condom use markedly lowers the risk of transmission.[17] The following table summarizes risk of HIV acquisition based on a number of factors related to a sexual exposure.[17] (Table 2)
Indications for Initiating HIV Nonoccupational PEP
Based on the 2025 HIV nPEP Recommendations, if the following criteria are met, a 28-day course of antiretroviral medications for HIV nonoccupational PEP is recommended:[5]
- A person has had a nonoccupational exposure to blood, genital secretions, or other potentially infectious body fluids from a person known to have HIV (if the HIV status of the source person is unknown, the recommendation is to evaluate on a case-by-case basis, ideally in consultation with an expert), and
- The exposure represents a substantial risk for HIV transmission, and
- The person seeks care within 72 hours of exposure.
When the source’s HIV status is unknown, administering HIV nonoccupational PEP can be considered on a case-by-case basis if the exposure presents a substantial risk for HIV transmission, and it occurred within the prior 72 hours. If the HIV status of the source is unknown, but they are available for HIV testing, the initiation of HIV nonoccupational PEP should not be delayed while determining the HIV status.[4]
Indications for HIV Nonoccupational PEP After Sexual Exposure to HIV
For a possible sexual exposure to HIV, multiple factors are taken into account if considering HIV nonoccupational PEP: when the exposure(s) occurred, type of sex, condom use during sex, HIV PrEP use by the person under evaluation, HIV status of source, and viral suppression in source.[4] If the source’s HIV status is unknown, then administering HIV nonoccupational PEP should occur on a case-by-case basis.[4] For sexual exposures, HIV nonoccupational PEP is not recommended if the person exposed to HIV is taking HIV PrEP as recommended.[4] In addition, with potential sexual exposures to HIV, the use of HIV nonoccupational PEP is not routinely recommended if the source person with HIV has documented sustained viral suppression.[4] The algorithm below summarizes guidance for administering HIV nonoccupational PEP following a potential sexual exposure to HIV (Figure 2).[4]
Indications for HIV Nonoccupational PEP After Injection Drug Exposure
For possible exposures to HIV in the setting of injection drug use, there are three main factors to consider when considering HIV nonoccupational PEP: the timeframe when the exposure(s) occurred, HIV status of source, and viral suppression in source.[4] If the source’s HIV status is unknown, then deciding whether to administer HIV nonoccupational PEP should occur on a case-by-case basis.[4] Similar to the recommendation listed above related to possible sexual exposure to HIV, the use of HIV nonoccupational PEP is not routinely recommended following an exposure to HIV associated with injection drug use if the source person has documented sustained viral suppression.[4] Unlike recommendations for possible sexual exposure to HIV, the recommendations for possible exposure to HIV in the setting of injection drug use do not factor in whether the person who was exposed to HIV is taking HIV PrEP; the rationale for not including HIV PrEP in this setting is that available data suggest HIV PrEP in persons who inject drugs is only approximately 74% effective.[4,39] The algorithm below summarizes recommendations for giving HIV nonoccupational PEP following a potential exposure to HIV in the setting of injection drug use (Figure 3).[4]
Indications for HIV Nonoccupational PEP After Possible Other Exposures
In addition to sexual and injection-drug-related potential exposures to HIV, individuals may seek evaluation and consideration of HIV nonoccupational PEP after exposures that pose a much lower risk of HIV transmission.[4] These types of exposures have varying risks and include blood or infective body fluid splash onto non-intact skin or mucous membranes, injury from a discarded needle in the community, human bites, kissing, mutual masturbation, and exposure to body fluids not associated with HIV transmission (e.g., tears, sweat, urine, nasal secretions, and saliva).[4] The algorithm below summarizes recommendations for the consideration of administering HIV nonoccupational PEP following these other types of exposures (Figure 4).[4]
Recommendation if Source Person Has Sustained Viral Suppression
Multiple studies have shown that persons with HIV who consistently maintain undetectable serum HIV RNA levels do not sexually transmit HIV, even with condomless sex.[40,41,42] Accordingly, for sexual exposures involving a source person who has sustained suppression of plasma HIV RNA levels, nonoccupational HIV PEP is not routinely recommended.[4] In this context, “sustained viral suppression” is stringently defined as HIV treatment longer than 6 months, a consistent high level of antiretroviral adherence, and HIV RNA less than 200 copies/mL or undetectable in all laboratory assessments in the last year, including an HIV RNA result within the prior 1–2 months.[4] From a practical standpoint, however, this level of detailed information about the source’s HIV RNA test results is often unknown, particularly HIV RNA test results in the prior 1–2 months. For exposures to HIV associated with injection drug use, the same recommendation exists—HIV nonoccupational PEP is not routinely recommended if the source person is known to have sustained viral suppression.[4] This recommendation is based on an extrapolation of data from studies on HIV RNA levels and risk of sexual transmission of HIV. Rigorous studies examining plasma HIV RNA levels and risk of HIV transmission through injection drug use have not been published.
Recommendation if Person Exposed to HIV is Taking HIV PrEP
Persons who are taking HIV PrEP (oral tenofovir DF-emtricitabine, oral tenofovir alafenamide-emtricitabine, injectable cabotegravir, and injectable lenacapavir injection) as recommended do not need HIV nonoccupational PEP following a possible sexual exposure to HIV.[4] This recommendation is based on extensive data showing very high protection against sexual acquisition of HIV when HIV PrEP is taken with high adherence. The 2025 HIV nPEP Recommendations do not provide an adherence cutoff that defines taking HIV PrEP as recommended.[4] In studies involving use of daily oral tenofovir-DF for HIV PrEP in men who have sex with men, adherence with at least 4 doses per week was estimated to provide a greater than 95% risk reduction for acquiring HIV.[43,44] Laboratory studies examining vaginal tissue levels of the active metabolites of tenofovir and lamivudine suggest that 6–7 doses per week (greater than 85% adherence) with oral HIV PrEP is required to achieve drug levels in lower vaginal tract tissues for significant protection against vaginal acquisition of HIV.[45] In a pooled analysis of 11 clinical studies that involved tenofovir DF-emtricitabine for HIV PrEP among women, individuals with consistent high adherence (4–7 doses per week) experienced very low HIV incidence.[46] For persons taking injectable HIV PrEP, adherence effectiveness data are limited, but presumably, persons who do not miss doses of injectable cabotegravir or lenacapavir would be expected to have very high protection against HIV. There are insufficient data on medication adherence and prevention of HIV for exposures related to injection drug use. Thus, the use of HIV PrEP is not factored in with decision-making after a nonoccupational exposure to HIV associated with injection drug use.[4]
Recommended Regimens for HIV Nonoccupational PEP
The 2025 HIV nPEP Recommendations endorse using a 3-drug combination antiretroviral regimen given for 28 days in all cases when HIV nonoccupational PEP is indicated.[5] The general recommendation to use a 3-drug regimen (versus a 1- or 2-drug regimen) for HIV nonoccupational PEP is based on the following factors: HIV treatment data that show more rapid and reliable suppression of HIV RNA levels with a 3-drug regimen, a 3-drug regimen would likely be more effective than a 1- or 2-drug if the source person had drug-resistant HIV, and the safety and tolerance of a 28-day course with the newer recommended 3-drug regimens is excellent.[4] The selection of the specific HIV nonoccupational PEP regimen should be individualized and should take into account potential drug interactions with concurrent medications, pregnancy, renal dysfunction, hepatic impairment, and cost.[4] In nearly all situations, a once-a-day regimen can be used for HIV nonoccupational PEP.
Time to Initiation of HIV Nonoccupational PEP
The initiation of HIV nonoccupational PEP, when indicated, should occur as soon as possible.[4] Ideally, the HIV nonoccupational PEP is started within 24 hours of the exposure, but initiation any time within 72 hours after the exposure is considered acceptable.[4] In certain circumstances, such as a very high risk exposure, some experts would consider offering HIV nonoccupational PEP if the exposure occurred more than 72 hours prior to the person seeking evaluation.[4]
Duration of Therapy
The 2025 HIV nPEP Recommendations recommend that individuals who initiate antiretroviral therapy for HIV nonoccupational PEP should complete a 28-day course.[4] Studies involving macaques have shown that HIV PEP given for 28 days is more effective than 10 days, which is more effective than 3 days.[13] In addition, available data and experience with occupational PEP support the use of a 28-day regimen.[8,47] From a conceptual standpoint, it is believed that HIV PEP, in some instances, halts a very early and limited HIV infection, rather than truly preventing any cell in the body from becoming infected with HIV. Thus, early initiation of HIV nonoccupational PEP, when combined with a 28-day duration of therapy, is believed adequate to minimize tissue involvement and contain any local HIV replication while allowing sufficient time for localized immune responses to clear out the limited HIV infection.
HIV Nonoccupational PEP Regimens for Adults and Adolescents
For persons 12 years of age and older who do not have renal dysfunction or hepatic impairment, the preferred regimens for nonoccupational PEP consist of an integrase strand transfer inhibitor plus two nucleoside reverse transcriptase inhibitors; the alternative regimen is a boosted protease inhibitor plus two nucleoside reverse transcriptase inhibitors(Table 3).[4]
HIV Nonoccupational PEP Regimens in Pregnant Women
Ideally, expert consultation is advised when considering HIV nonoccupational PEP for pregnant or breastfeeding women. The preferred and alternative regimens for pregnant women are the same when administering HIV nonoccupational PEP for persons 12 years of age and older who are not pregnant, except that when darunavir is used in pregnant women, it should be used only with ritonavir boosting and both darunavir and ritonavir should be given twice daily instead of the usual once-daily dosing (Table 4).[4]
HIV Nonoccupational PEP Regimens in Women who are Breastfeeding
There are limited data on the use of HIV nonoccupational PEP in women who are breastfeeding. If a woman has an exposure to HIV and is breastfeeding, HIV nonoccupational PEP should be offered to her, if indicated, based on the exposure. The HIV nonoccupational PEP regimens should be the same as those used for persons older than 12 years of age who are not pregnant (e.g., if darunavir is used, it should be dosed once daily with either cobicistat or ritonavir. Ideally, expert consultation is advised when considering HIV nonoccupational PEP for breastfeeding women. If a woman who is breastfeeding is going to start HIV nonoccupational PEP, counseling should include a discussion and shared decision-making regarding whether to interrupt breastfeeding during the 28-day course of HIV nonoccupational PEP.
HIV Nonoccupational PEP Regimens for Children and Adolescents
In many pediatric nonoccupational HIV PEP cases, expert consultation should be obtained due to unique medication dosing adjustments that may be needed in children based on weight and age, as well as to provide an in-depth examination of the circumstances of the exposure that led to the evaluation for HIV nonoccupational PEP, especially when there is concern for sexual assault. The following summarizes recommendations in the 2025 HIV nPEP Recommendations for children up to 12 years of age (Table 5).[4]
- Children ≥2 Years to 12 Years: The recommended HIV nonoccupational PEP regimens or children aged 2 to 12 years are the same as those for adolescents and adults, but with four differences: (1) bictegravir-tenofovir alafenamide-emtricitabine is not recommended for children who weigh less than 14 kg; (2) darunavir should only be used in children 3 years of age and older who weigh at least 10 kg and when darunavir is used, it should be boosted with ritonavir and not cobicistat; (3) lopinavir-ritonavir can be used in place of darunavir plus ritonavir in the alternative regimens that use a boosted protease inhibitor plus two nucleoside reverse transcriptase inhibitors; and (4) tenofovir alafenamide-emtricitabine should not be used with a boosted protease inhibitor if the child’s weight is less than 35 kg.
- Children ≥4 Weeks to 2 Years: The preferred regimen consists of the integrase strand transfer inhibitor dolutegravir plus two nucleoside reverse transcriptase inhibitors (zidovudine plus [emtricitabine or lamivudine]). In the alternative regimens, dolutegravir is replaced with either raltegravir or lopinavir (boosted with ritonavir).
- Neonates Aged ≥14 Days to <4 Weeks: The need for HIV nonoccupational PEP in this age group should be exceedingly rare. If there is a possible need for HIV nonoccupational PEP in this age group, all management should be in conjunction with expert consultation.
HIV Nonoccupational PEP Regimens in Patients with Renal Dysfunction or Hepatic Insufficiency
For adults and adolescents with renal or hepatic impairment, some adjustments may be needed in the recommended HIV nonoccupational PEP regimens (Table 6).[4]
- Patients with Renal Insufficiency: For persons with moderate renal insufficiency (CrCl 30-49 mL/min), the major adjustment is that tenofovir DF is not part of any preferred regimen, and when used as part of an alternative regimen, dose-reduced tenofovir DF is used (adjusted to 300 mg every 48 hours).[4] For persons with severe renal insufficiency (CrCl less than 30 mL/min) who are receiving hemodialysis, the same options can be used as with moderate renal insufficiency, but use of tenofovir DF and/or lamivudine requires dose adjustment.[4] The management of persons with severe renal insufficiency who are not on dialysis is highly complicated and generally warrants expert consultation.[4]
- Patients with Hepatic Insufficiency: For individuals who have hepatic impairment with Child-Pugh class A or B, the same preferred and alternative nonoccupational PEP regimens can be used as recommended for persons without hepatic impairment.[4] For individuals with severe hepatic impairment (Child-Pugh class C), expert consultation should be obtained to choose an appropriate nonoccupational PEP regimen.[4]
Expert Consultation for HIV Nonoccupational PEP
Indications for Obtaining Expert Consultation
The 2025 HIV nPEP Recommendations provide recommendations for scenarios that warrant expert consultation for HIV nonoccupational PEP.[4] Expert consultation is recommended in any of the following situations:
- The health care worker has limited experience with prescribing antiretroviral medications, or
- The individual exposed to HIV is a woman who is pregnant or breastfeeding, or
- The exposure event involves a child or adolescent, or
- The individual needing HIV nonoccupational PEP has severe renal dysfunction and is not on hemodialysis, or
- The individual needing HIV nonoccupational PEP has severe hepatic impairment (Child-Pugh class C), or
- The source person has known or suspected antiretroviral resistance.
Consideration of HIV Resistance in Source Person
If medical information is available regarding a source person known to have HIV, the choice of an HIV nonoccupational PEP regimen should take into account the source person’s antiretroviral medication history, most recent HIV RNA levels, and prior resistance testing results.[4] Drug resistance to the newer generation integrase strand transfer inhibitors (bictegravir and dolutegravir) is uncommon.[4] In the event the source person has suspected or known antiretroviral drug resistance, expert consultation should be obtained to determine the optimal HIV nonoccupational PEP regimen for the individual exposed to HIV.[4]
PEPline Expert Consultation
Expert consultation for these issues and any other guidance on nonoccupational PEP can be obtained by calling the National Clinician Consultation Center’s Post-Exposure Prophylaxis PEPline at 844-275-6222; this service is for health care professionals.
Baseline and Follow-Up Laboratory Testing
Performing baseline laboratory evaluation and follow-up laboratory studies is an important component of nonoccupational HIV PEP evaluation and management. Testing for HIV is the most important test for the baseline and follow-up laboratory evaluation. For the person possibly exposed to HIV, additional important laboratory studies include testing for viral hepatitis (hepatitis B virus [HBV] and hepatitis C virus [HCV]), sexually transmitted infections for sexual exposures (syphilis, gonorrhea, and chlamydia), serum creatinine, hepatic aminotransferase levels, and a baseline pregnancy test (for women of childbearing potential, unless they are known to be pregnant).[4] Follow-up laboratory testing for the person exposed to HIV is typically performed 4–6 weeks and 12 weeks after the initial evaluation, with additional testing at 6 months if follow-up for HBV or HCV infection is warranted.[4] As outlined in the following table and discussion below, the 2025 HIV nPEP Recommendations provide extensive recommendations for baseline laboratory studies (for the source and the person exposed to HIV), as well as a schedule of follow-up laboratory tests for monitoring the person exposed to HIV.[4] (Table 7)
Baseline Laboratory Evaluation
Baseline HIV testing of the person undergoing evaluation for HIV nonoccupational PEP should always include HIV testing.[4] In addition, the source person should have HIV testing, unless the source is not available to undergo testing or the source is already known to have HIV.[4] Unfortunately, with many nonoccupational exposures to HIV, information about the source’s HIV status is not known. The following summarizes key information regarding baseline laboratory testing for both the source and the person exposed to HIV; the laboratory evaluation is very similar for the source and the person exposed, except for three differences: (1) HCV RNA testing is recommended for source whereas HCV antibody with reflex to HCV RNA is recommended for person exposed to HIV; (2) pregnancy testing is considered only for the person exposed; and (3) baseline serum creatinine and hepatic aminotransferase levels are recommended only for person exposed.[4]
- HIV Testing: Baseline HIV testing for the source and the person possibly exposed to HIV should consist of a rapid (point-of-care) test, a laboratory-based HIV antigen-antibody test, or both. If a point-of-care HIV test is used, a laboratory-based HIV antigen-antibody test should also be ordered, primarily because of the higher sensitivity of the laboratory-based HIV antigen-antibody tests. Oral point-of-care HIV tests are not recommended. Some experts recommend obtaining a baseline diagnostic HIV nucleic acid test (NAT) for the source or the individual under evaluation if they have recently taken oral HIV PrEP medications, or they received an injectable HIV PrEP medication (cabotegravir or lenacapavir) in the prior 12 months.
- Serologic Testing for Hepatitis B Virus (HBV): The source and the person exposed should undergo an HBV triple screen that consists of hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), and hepatitis B core antibody (HBcAb). Baseline screening is indicated to determine whether nonoccupational PEP against HBV may be indicated (the source person has a positive HBsAg test, and the person with the exposure is not immune to HBV). In addition, it is important to identify if the person starting HIV nonoccupational PEP has chronic HBV, since starting one or more medications that have activity against hepatitis B (tenofovir DF, tenofovir alafenamide, emtricitabine, or lamivudine) can lead to a hepatic flare after discontinuation. Further, for a person who lacks immunity to HBV and does not have active HBV infection, immunization against HBV is recommended.
- Hepatitis C Virus (HCV): For the source person, HCV RNA NAT testing is recommended. For the person with a possible exposure to HIV, the recommended testing is HCV antibody with reflex to HCV RNA if the antibody test is reactive. Testing the source and the person exposed is indicated to determine whether the exposure has the potential for HCV transmission. Although nonoccupational PEP against HCV is not recommended, follow-up for a person exposed to HCV is very important since, in the event they acquire HCV, highly effective and safe treatments for HCV are available.
- Sexually Transmitted Infections: Baseline testing for sexually transmitted infections (syphilis, gonorrhea, and chlamydia) should be individualized based on the exposure, as well as any other indication for screening. Most experts recommend routine testing for syphilis, gonorrhea, and chlamydia in the setting of a possible sexual exposure to HIV. Ideally, baseline testing for sexually transmitted infection is performed on both the source and the person possibly exposed to HIV. In this setting, the evaluation for sexually transmitted infections should include serologic testing for syphilis and nucleic acid amplification tests (NAATs) for chlamydia and gonorrhea (at all exposure sites). If presumptive treatment for these infections is not administered (to the person with the exposure), repeat testing can be ordered approximately 2 weeks later. In addition, for women with a possible sexual exposure to HIV, some experts recommend baseline testing for trichomonas (using a urine or vaginal swab sample).
- Pregnancy Test: Although all medications that are recommended for HIV nonoccupational PEP are considered safe in pregnancy and during breastfeeding, additional counseling may be needed. Therefore, pregnancy testing is recommended for women with childbearing potential who are undergoing evaluation for HIV nonoccupational PEP.
- Serum Creatinine: This test is recommended for persons exposed to HIV who are starting an HIV nonoccupational PEP regimen. The rationale for checking a baseline serum creatinine is twofold: (1) tenofovir DF and tenofovir alafenamide have the potential to cause small, reversible declines in renal function, and (2) nonoccupational PEP medication regimens or dose adjustments may be needed with moderate or severe renal dysfunction.
- Hepatic Aminotransferase Levels: Testing for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels is recommended for persons exposed to HIV who are starting an HIV nonoccupational PEP regimen. Liver injury from nonoccupational PEP medication regimens is extremely rare, but dose adjustments may be needed with severe hepatic impairment (Child-Pugh class C).
Follow-Up Laboratory Studies
The 2025 HIV nPEP Recommendations recommend the following laboratory tests for all persons who received HIV nonoccupational PEP.[5] In addition, follow-up laboratory studies are recommended for individuals with a significant exposure to HIV who declined HIV nonoccupational PEP.
- HIV Testing: Follow-up HIV testing should consist of a laboratory-based HIV antigen-antibody test in combination with an HIV RNA NAT, with the testing performed at 4–6 weeks and 12 weeks after the baseline evaluation. If the person started HIV nonoccupational PEP within 24 hours of the exposure and was adherent with the entire 28-day regimen, the testing at 4–6 weeks can be omitted. If the person is planning to transition from nonoccupational HIV PEP to HIV PrEP, then the first follow-up and testing would ideally occur exactly at 4 weeks (or a day or two prior to 4 weeks) to coordinate the switch from HIV nonoccupational PEP to HIV PrEP.
- HBV Testing: For persons not immune to HBV at baseline, follow-up HBV testing should be conducted at 6 months and should include HBsAg, HBsAb, and HBcAb.
- HCV Testing: If the source has a positive HCV RNA test (or a positive HCV antibody with unknown HCV RNA results), follow-up HCV antibody testing is recommended for the person exposed to HIV at 6 months.
- Testing for Sexually Transmitted Infections: Routine follow-up testing for syphilis, gonorrhea, and chlamydia is not recommended. If, however, the person is diagnosed with a sexually transmitted infection at baseline, or they are transitioning to HIV PrEP, then follow-up testing for sexually transmitted infections should be performed.
- Pregnancy Testing: Repeat pregnancy testing should be performed at 4–6 weeks if the woman is of reproductive age and not known to be pregnant.
- Serum Creatinine: A serum creatinine should be checked at 4–6 weeks only if the serum creatinine was abnormal at baseline.
- Hepatic Aminotransferase Levels: The ALT and AST levels should be checked at 4–6 weeks if the hepatic aminotransferase levels were abnormal at baseline.
HIV Nonoccupational PEP After Sexual Assault
The evaluation and provision of HIV nonoccupational PEP should be part of comprehensive services offered to persons who have experienced sexual assault. Extensive efforts should be made for these services to include a comprehensive evaluation by a sexual assault forensic or nurse examiner, as well as services or referral for psychological counseling. It is beyond the scope of this topic review to address the extensive issues, including legal issues, that need to be addressed following a sexual assault of an adult, adolescent, or child, but these recommendations can be found in published guidelines, including the American College of Obstetricians and Gynecologists (ACOG) Committee Opinion No. 777: Sexual Assault, the American Academy of Pediatrics, the CDC Sexually Transmitted Infections Treatment Guidelines section Sexual Assault and Abuse and STIs.[48,49,50] The following will focus on key postexposure medical interventions following a sexual assault.
Emergency Contraception
When a sexual assault victim is a woman and the assault has the possibility of resulting in pregnancy, emergency contraception should be offered.[4,48]
HIV Nonoccupational PEP
Baseline testing for HIV is recommended for all persons who are evaluated after a sexual assault.[4,48] In addition, follow-up HIV testing is recommended at 4–6 weeks and 12 weeks after the sexual assault.[4,48] In most cases of sexual assault, information on the source’s HIV status is not known, and if known, detailed knowledge of recent viral load data is unlikely. Nevertheless, for sexual assault survivors, HIV nonoccupational PEP should be offered if the assault involved contact associated with substantial risk of HIV transmission and the source is known to have HIV infection (or the source’s HIV status is unknown).[4] Available data suggest low adherence and low completion rates for HIV nonoccupational PEP among sexual assault survivors.[51,52] Therefore, at the initial visit, it is important to provide counseling and education on the importance of taking the entire 28-day regimen, as well as provide adherence support. Multiple studies involving sexual assault survivors have demonstrated very low HIV transmission rates, despite relatively low rates of adherence with HIV nonoccupational PEP medications.[53,54,55,56,57]
Empiric Treatment for Common Sexually Transmitted Infections
Baseline testing for all persons following sexual assault should include NAATs for C. trachomatis and N. gonorrhoeae at all sites of penetration.[48] All sexual assault survivors should have baseline serologic testing for syphilis; if a syphilis diagnosis is not ruled out in the assailant, follow-up syphilis serologic testing should be conducted at 4–6 weeks and 3 months, which coincides with the timing of follow-up HIV testing.[4,48] Women should also have a NAAT for Trichomonas vaginalis from a urine or vaginal specimen.[48] Empiric treatment for gonorrhea, chlamydia, and trichomoniasis should be offered to all women following a sexual assault.[48] For pregnant women, azithromycin should be used in place of doxycycline. Men should have empiric treatment for gonorrhea and chlamydia. Empiric initial treatment for STIs should be provided, regardless of whether the test results for these pathogens are available. Note that empiric therapy for syphilis is not routinely recommended in sexual assault survivors, but ceftriaxone and doxycycline both have significant activity against Treponema pallidum and may provide some protection against incubating syphilis.
Postexposure Prophylaxis for HBV
The indication for HBV postexposure prophylaxis depends on the prior HBV vaccine history and immune status of the sexual assault survivor and information that is available regarding the assailant’s HBV status, if known. The recommendations for HBV nonoccupational PEP are provided in the section Nonoccupational PEP for Infectious Pathogens Other than HIV.
Postexposure Prophylaxis for HCV
There are no recommendations to provide HCV postexposure prophylaxis, but baseline HCV antibody testing is recommended, with reflex to HCV RNA if reactive. In addition, if indicated, follow-up HCV RNA testing should be performed, with an HCV RNA at 3–6 weeks after exposure and an HCV antibody (with reflex to HCV RNA) performed at 4-6 months postexposure.
Human Papillomavirus (HPV) Vaccination
For survivors of sexual assault who are 9–26 years of age, the human papillomavirus (HPV) vaccine should be administered to those individuals who have not previously received the HPV vaccine series.[48] For healthy persons 9–14 years of age, two doses of the vaccine are recommended. For all others, the vaccine should be administered as a 3-dose series. For individuals who have started the vaccine series but not completed it, they should complete the vaccine series based on their age when they started the series.
Herpes Simplex Virus
A sexual assault has the potential to result in transmission of herpes simplex virus (HSV) type 1 and/or type 2 from the assailant to the sexual assault survivor. Although there are no recommendations to perform HSV testing or to provide HSV postexposure prophylaxis following sexual assault, clinicians should be aware of this possible complication.
Initial Medication Prescription and Follow-up after Evaluation
Initial Medication Prescription and Timely Follow-Up
The 2025 HIV nPEP Recommendations recommend that medical providers should consider giving an initial prescription of antiretroviral medications for 3 to 7 days (i.e., a starter pack) or provide a prescription for an entire 28-day course for HIV nonoccupational PEP. Ideally, at the initial visit, the facility would supply the starter pack medication or the full 28-day supply of medication, both to minimize any delay in receiving the first dose and to address any barriers that could prohibit the patient from filling the prescription. In addition, prior to leaving the facility, the person evaluated for HIV nonoccupational PEP should have an early follow-up visit scheduled or a phone call check-in to assess adherence with HIV nonoccupational PEP, discuss any medication-related side effects, and provide any additional counseling or education that might be needed.[4] If the individual receives only a 3- to 7-day supply of antiretroviral medications, coordination of timely follow-up is essential to ensure they do not run out of medication.
Challenges to Follow-Up
One study reported significant patient attrition between the initial emergency department visit for HIV nonoccupational PEP and the first follow-up clinic appointment.[58] Only about half of HIV nonoccupational PEP patients attended their follow-up appointments, and less than a quarter of those who initially started on nonoccupational HIV PEP completed the full 28-day regimen.[58]
HIV Prevention Counseling
Patients who are evaluated for nonoccupational HIV PEP should receive HIV prevention counseling. This includes counseling on methods to reduce the risk of HIV acquisition (e.g., using a barrier method with sex partners, not sharing equipment used to inject drugs). At follow-up visits, health care providers should assess for ongoing risk activities, provide additional counseling, and connect patients with services as needed, including assessment for initiation of HIV PrEP.
Transitioning from HIV Nonoccupational PEP to HIV PrEP
Individuals who present for HIV nonoccupational PEP following a sexual or injection drug use exposure may be excellent candidates for HIV PrEP, particularly if they report ongoing activities associated with increased risk for HIV acquisition.[6,59] Thus, persons receiving HIV nonoccupational PEP who are interested in HIV PrEP should transition from HIV nonoccupational PEP to HIV PrEP without a gap.[4,6] For example, if a person is going to transition from HIV nonoccupational PEP to HIV PrEP, they should take a 28-day course of HIV nonoccupational PEP, and on day 29, transition to taking HIV PrEP.[4,6] The following summarizes several key issues regarding the transition from HIV nonoccupational PEP to HIV PrEP.[6]
- The recommended first follow-up visit for any person initiating HIV nonoccupational PEP is 4–6 weeks. For persons who are candidates to transition from HIV nonoccupational PEP to HIV PrEP, this first follow-up visit should ideally be performed at 4 weeks (at the completion of the 28-day HIV nonoccupational PEP regimen or ideally even several days prior to completing the regimen). At this 4-week follow-up visit, repeat HIV testing should be performed, with a laboratory-based HIV antigen-antibody test and an HIV diagnostic NAT. There is an increased chance of false-negative HIV testing results in persons receiving HIV nonoccupational PEP, since potent antiretroviral therapy may suppress the normal immune reaction to HIV and may fully suppress plasma HIV RNA levels. At this visit, the individual should have an assessment for any signs or symptoms that would suggest acute HIV.
- At the 4-week visit, individuals transitioning to HIV PrEP should receive counseling about HIV PrEP, obtain the HIV PrEP prescription, and understand the need for regular follow-up visits and laboratory studies. The person can transition to HIV PrEP at the 28-day visit while the HIV results are pending, with the plan to immediately convert the HIV PrEP to HIV treatment if the HIV testing reveals HIV infection. If any concern for acute HIV exists, then HIV PrEP should be deferred while evaluation for acute HIV is completed.
- The individual taking a 3-drug HIV nonoccupational PEP regimen can transition to any of the HIV PrEP regimens (tenofovir DF-emtricitabine, tenofovir alafenamide-emtricitabine, long-acting injectable cabotegravir, or injectable lenacapavir), as long as the HIV PrEP regimen is indicated for the individual.
- For men who have sex with men, doxycycline PEP (Doxy PEP) should be discussed as an option for prevention of bacterial sexually transmitted infection.[60] At this time, Doxy PEP is not recommended for women.[60]
Expert Consultation for Transitioning from HIV Nonoccupational PEP to HIV PrEP
Clinicians with questions about transitioning from HIV nonoccupational PEP to HIV PrEP can call the National Clinician Consultation Center’s Pre-Exposure Prophylaxis PrEPline at 855-448-7737 for expert consultation.
Nonoccupational PEP for Infectious Pathogens Other Than HIV
In conjunction with HIV nonoccupational PEP management, consideration should also be given to evaluation and management for other infectious pathogens that can be transmitted as a result of the exposure. The risk for certain pathogens depends on whether the exposure involved sexual contact or injection drug use. For example, the management of potential transmission of T. pallidum, N. gonorrhoeae, and C. trachomatis is more important following a nonoccupational sexual exposure than an exposure related to injection drug use. In contrast, transmission of HBV or HCV is more likely with an injection drug use-related exposure than with a sexual exposure. The following outlines some key additional considerations for pathogens other than HIV that can be transmitted as a result of a sexual or injection-drug-related exposure. Additional details and recommendations for sexual assault survivors are provided in this lesson in the section on HIV Nonoccupational PEP after Sexual Assault.
Hepatitis B Virus
Hepatitis B virus is highly infectious and transmission may occur through blood contact via percutaneous routes (e.g., injection drug use), mucosal sexual contact (with exchange of blood, semen, or vaginal secretions), and non-intact skin exposure to infectious blood or body fluids.[61] Transmission in adults primarily occurs through percutaneous exposure to blood (e.g., by injection drug use) and sexual contact.[61] The risk of HBV transmission related to blood contact is substantially higher than the risk of transmission with sexual contact. Rare cases of HBV transmission have occurred with a bite wound. The risk of acquiring HBV depends on the type of exposure, the HBsAg status (if known) of the source person, and the HBV status of the person with the nonoccupational exposure. The strategy for preventing HBV acquisition via occupational, nonoccupational, and perinatal routes has utilized administration of the HBV vaccine and/or hepatitis B immune globulin (HBIG). For persons receiving HIV nonoccupational PEP, the antiretroviral regimen includes nucleoside reverse transcriptase inhibitors (tenofovir DF, tenofovir alafenamide, emtricitabine, and lamivudine) that have activity against HBV. The impact of using two of these nucleoside reverse transcriptase inhibitors (e.g., tenofovir DF-emtricitabine or tenofovir alafenamide-emtricitabine) on preventing HBV acquisition is unknown. Therefore, current HBV nonoccupational PEP recommendations, as outlined in the table below, utilize only the HBV vaccine and/or HBIG (Table 9).[61]
Hepatitis C Virus
Most cases of HCV infection in the United States have resulted from injection drug use.[62] Less often, transmission of HCV can occur through sexual contact, usually with cases involving men who have sex with men (MSM).[62] All persons who are evaluated for HIV nonoccupational PEP should have baseline HCV antibody testing, with reflex to HCV RNA NAT if positive.[4] For the person who had the nonoccupational exposure, follow-up HCV testing should be performed if indicated based on the source’s HCV status (e.g., HCV RNA NAT positive or positive HCV antibody with unavailable HCV RNA NAT, or if the HCV infection status is unknown).[4] If indicated, the follow-up HCV testing should consist of an HCV RNA NAT at 3–6 weeks postexposure and a final test for HCV antibodies (with reflex to HCV RNA NAT if positive) at 4–6 months postexposure.[4] There are no recommendations to use HCV direct-acting antiviral (DAA) therapy for nonoccupational PEP, but for persons diagnosed with HCV infection, DAA therapy can provide cure rates of greater than 95%.[4,63] Accordingly, baseline and follow-up HCV testing is an important aspect of the overall nonoccupational PEP evaluation to identify anyone who has HCV infection so they can receive curative antiviral therapy.
Syphilis
For persons under evaluation for HIV nonoccupational PEP, baseline serologic testing for syphilis should be made on an individual basis.[4] With an HIV nonoccupational exposure that occurs via sexual contact, most experts would recommend doing baseline and follow-up syphilis serologic testing. If the exposure involved sexual assault, baseline syphilis serologic testing should be ordered. There are no recommendations to routinely provide empiric treatment for syphilis, even following sexual assault. For men who have sex with men, doxycycline PEP (a single 200-mg dose taken within 72 hours of the exposure) could be offered and would be expected to provide moderate protection against syphilis.[60] At this time, doxycycline PEP is not recommended for women.
Gonorrhea
For persons under evaluation for HIV nonoccupational PEP, baseline testing for gonorrhea should be made on an individual basis.[4] With an HIV nonoccupational exposure that occurs via sexual contact, most experts would recommend doing baseline and follow-up testing for gonorrhea; testing (NAAT) should be done at all exposure sites (e.g., vagina, rectum, pharynx). There are no recommendations to routinely provide empiric treatment for gonorrhea in this setting, but for men who have sex with men, doxycycline PEP (a single 200-mg dose taken within 72 hours of the exposure) could be offered and would be expected to provide moderate protection against gonorrhea.[60] If the exposure involved sexual assault, empiric treatment with a single 500 mg intramuscular dose of ceftriaxone is recommended.[48]
Chlamydia
For persons under evaluation for HIV nonoccupational PEP, baseline testing for chlamydia should be made on an individual basis.[4] With an HIV nonoccupational exposure that occurs via sexual contact, most experts would recommend doing baseline and follow-up testing for gonorrhea. Testing (NAAT) should be done at all exposure sites (e.g., vagina, rectum, pharynx). There are no recommendations to routinely provide empiric treatment for gonorrhea in this setting, but for men who have sex with men, doxycycline PEP (a single 200-mg dose taken within 72 hours of the exposure) could be offered and would be expected to provide moderate protection against chlamydia.[60] If the exposure involved sexual assault, empiric treatment for chlamydia with oral doxycycline (100 mg twice daily for 7 days) is recommended, unless the assault involved a woman who was pregnant.[48] For pregnant women, empiric treatment with azithromycin 1 gram orally is recommended.[48]
Trichomonas
Baseline testing for trichomoniasis is not routinely recommended for persons under evaluation for HIV nonoccupational PEP. Some experts recommend testing women following a sexual exposure with a NAAT for Trichomonas vaginalis in a urine or vaginal specimen. There are no recommendations to routinely provide empiric treatment for trichomoniasis for persons evaluated for HIV nonoccupational PEP. If, however, the exposure involved sexual assault, empiric treatment of trichomoniasis for women should be offered with oral metronidazole (500 mg twice a day for 7 days).[48]
Herpes Simplex Virus
There are no recommendations to perform testing or empiric treatment for HSV following a nonoccupational sexual or injection-drug-related exposure. Nevertheless, clinicians should be aware that transmission of HSV-1 and/or HSV-2 could occur with a sexual exposure.
Human PapillomaVirus
There are no recommendations to perform testing for human papillomavirus (HPV) with HIV nonoccupational PEP evaluations. For persons who have not received HPV immunization, the HPV vaccine series should be offered. For survivors of sexual assault who are 9–26 years of age, the HPV vaccine should be administered to those individuals who have not previously received the HPV vaccine series. It is unknown whether promptly initiating and then completing the HPV vaccine series provides any protection if given after a sexual exposure to HPV.
Summary Points
- The use of antiretroviral medications for HIV nonoccupational PEP is recommended for HIV-seronegative persons following an exposure that has a substantial risk for HIV acquisition, if started within 72 hours of the exposure. The optimal timing for administration of HIV nonoccupational PEP is within 24 hours of the exposure.
- For sexual exposures to HIV involving persons taking HIV PrEP as recommended, HIV nonoccupational PEP is not indicated. In addition, HIV nonoccupational PEP is not routinely recommended when the source with HIV is known to have sustained viral suppression. If the HIV status of the source is unknown, the use of HIV nonoccupational PEP should be evaluated on a case-by-case basis.
- A 28-day course of three-drug antiretroviral medications is recommended for HIV nonoccupational PEP.
- The preferred HIV nonoccupational PEP regimen for adults and adolescents aged 12 years and older consists of either the fixed-dose combination bictegravir-tenofovir alafenamide-emtricitabine or the regimen dolutegravir plus (tenofovir DF or tenofovir alafenamide) plus (emtricitabine or lamivudine). The preferred regimens for HIV nonoccupational PEP are safe and usually well tolerated.
- Regimens for HIV nonoccupational PEP may need to be modified in persons with significant renal dysfunction or severe hepatic impairment.
- Individuals who have a possible nonoccupational exposure to HIV should have a baseline laboratory evaluation that includes HIV testing (HIV antigen-antibody test), serum creatinine, AST, ALT, HBV, and a pregnancy test (if indicated). If a person has received long-acting injectable HIV PrEP in the prior 12 months, then baseline testing should include a diagnostic HIV NAT.
- For the person possibly exposed to HIV, baseline testing for sexually transmitted infections and HCV infection should be guided by the clinical situation.
- Expert consultation should be sought for complex situations and can be obtained through local expertise (if available) or through the National Clinician Consultation Center.
- Individuals who seek care for HIV nonoccupational PEP should have follow-up HIV testing at 4 to 6 weeks and again at 3 months to determine if HIV infection has occurred. The follow-up HIV testing should consist of the HIV-1/2 antigen-antibody test in tandem with a diagnostic HIV NAT.
- Many individuals who seek HIV nonoccupational PEP services should be evaluated as potential candidates to receive HIV PrEP following completion of HIV nonoccupational PEP. The transition from HIV nonoccupational PEP to HIV PrEP, if warranted, should occur without a gap.
Citations
- 1.Gerberding JL. Management of occupational exposures to blood-borne viruses. N Engl J Med. 1995;332:444-51.[PubMed Abstract] -
- 2.Centers for Disease Control and Prevention (CDC). Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR Morb Mortal Wkly Rep. 1996;45:468-80.[PubMed Abstract] -
- 3.Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54(No. RR-2):1-20.[CDC] -
- 4.Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.[PubMed Abstract] -
- 5.Dominguez KL, Smith DK, Thomas V, et al. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2016.[CDC] -
- 6.Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 Update: a clinical practice guideline. December 2021:1-108.[CDC] -
- 7.Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;41:1507-13.[PubMed Abstract] -
- 8.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485-90.[PubMed Abstract] -
- 9.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217-28.[PubMed Abstract] -
- 10.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795-802.[PubMed Abstract] -
- 11.Taha TE, Li Q, Hoover DR, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr. 2011;57:319-25.[PubMed Abstract] -
- 12.Woldesenbet S, Jackson D, Lombard C, et al. Missed Opportunities along the Prevention of Mother-to-Child Transmission Services Cascade in South Africa: Uptake, Determinants, and Attributable Risk (the SAPMTCTE). PLoS One. 2015;10:e0132425.[PubMed Abstract] -
- 13.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265-73.[PubMed Abstract] -
- 14.Otten RA, Smith DK, Adams DR, et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol. 2000;74:9771–5.[PubMed Abstract] -
- 15.Irvine C, Egan KJ, Shubber Z, Van Rompay KK, Beanland RL, Ford N. Efficacy of HIV Postexposure Prophylaxis: Systematic Review and Meta-analysis of Nonhuman Primate Studies. Clin Infect Dis. 2015;60 Suppl 3:S165-9.[PubMed Abstract] -
- 16.Crawford ND, Vlahov D. Progress in HIV reduction and prevention among injection and noninjection drug users. J Acquir Immune Defic Syndr. 2010;55 Suppl 2:S84-7.[PubMed Abstract] -
- 17.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509-19.[PubMed Abstract] -
- 18.Sonder GJ, Prins JM, Regez RM, et al. Comparison of two HIV postexposure prophylaxis regimens among men who have sex with men in Amsterdam: adverse effects do not influence compliance. Sex Transm Dis. 2010;37:681-6.[PubMed Abstract] -
- 19.Schechter M, do Lago RF, Mendelsohn AB, Moreira RI, Moulton LH, Harrison LH; for the Praca Onze Study Team. Behavioral Impact, Acceptability, and HIV Incidence Among Homosexual Men With Access to Postexposure Chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. 2004;35:519-25.[PubMed Abstract] -
- 20.Kahn JO, Martin JN, Roland ME, et al. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: the San Francisco PEP Study. J Infect Dis. 2001;183:707-14.[PubMed Abstract] -
- 21.Gulholm T, Jamani S, Poynten IM, Templeton DJ. Non-occupational HIV post-exposure prophylaxis at a Sydney metropolitan sexual health clinic. Sex Health. 2013;10:438-41.[PubMed Abstract] -
- 22.Pierce AB, Yohannes K, Guy R, et al. HIV seroconversions among male non-occupational post-exposure prophylaxis service users: a data linkage study. Sex Health. 2011;8:179-83.[PubMed Abstract] -
- 23.Fletcher JB, Rusow JA, Le H, Landovitz RJ, Reback CJ. High-risk Sexual Behavior is Associated with Post-Exposure Prophylaxis Non-adherence among Men who have Sex with Men Enrolled in a Combination Prevention Intervention. J Sex Transm Dis. 2013;2013:210403.[PubMed Abstract] -
- 24.Jain S, Oldenburg CE, Mimiaga MJ, Mayer KH. Subsequent HIV infection among men who have sex with men who used non-occupational post-exposure prophylaxis at a Boston community health center: 1997-2013. AIDS Patient Care STDS. 2015;29:20-5.[PubMed Abstract] -
- 25.Rey D, Bendiane MK, Bouhnik AD, Almeda J, Moatti JP, Carrieri MP. Physicians' and patients' adherence to antiretroviral prophylaxis after sexual exposure to HIV: results from South-Eastern France. AIDS Care. 2008;20:537-41.[PubMed Abstract] -
- 26.Liu A, Xin R, Zhang H, et al. An open-label evaluation of safety and tolerability of coformulated bictegravir/emtricitabine/tenofovir alafenamide for post-exposure prophylaxis following potential exposure to human immunodeficiency virus-1. Chin Med J (Engl). 2022;135:2725-9.[PubMed Abstract] -
- 27.Mayer KH, Gelman M, Holmes J, Kraft J, Melbourne K, Mimiaga MJ. Safety and Tolerability of Once Daily Coformulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide for Postexposure Prophylaxis After Sexual Exposure. J Acquir Immune Defic Syndr. 2022;90:27-32.[PubMed Abstract] -
- 28.McAllister JW, Towns JM, Mcnulty A, et al. Dolutegravir with tenofovir disoproxil fumarate-emtricitabine as HIV postexposure prophylaxis in gay and bisexual men. AIDS. 2017;31:1291-5.[PubMed Abstract] -
- 29.Gantner P, Allavena C, Duvivier C, et al. Post-exposure prophylaxis completion and condom use in the context of potential sexual exposure to HIV. HIV Med. 2020;21:463-9.[PubMed Abstract] -
- 30.Nie J, Sun F, He X, et al. Tolerability and Adherence of Antiretroviral Regimens Containing Long-Acting Fusion Inhibitor Albuvirtide for HIV Post-Exposure Prophylaxis: A Cohort Study in China. Infect Dis Ther. 2021;10:2611-23.[PubMed Abstract] -
- 31.Hutton L, MacPherson P, Corace K, Leach T, Giguere P. Tolerability of darunavir/ritonavir, tenofovir/emtricitabine for human immunodeficiency virus postexposure prophylaxis. Can J Hosp Pharm 2015;68:84.
- 32.Ford N, Shubber Z, Calmy A, et al. Choice of antiretroviral drugs for postexposure prophylaxis for adults and adolescents: a systematic review. Clin Infect Dis. 2015;60 Suppl 3:S170-6.[PubMed Abstract] -
- 33.Royce RA, Sena A, Cates W Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072-8.[PubMed Abstract] -
- 34.Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019;321:451-2.[PubMed Abstract] -
- 35.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Antiretroviral therapy to prevent sexual transmission of HIV (treatment as prevention). December 18, 2019.[HIV.gov] -
- 36.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118-29.[PubMed Abstract] -
- 37.Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol. 1998;148:88-96.[PubMed Abstract] -
- 38.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29:38-43.[PubMed Abstract] -
- 39.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083-90.[PubMed Abstract] -
- 40.Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5:e438-e447.[PubMed Abstract] -
- 41.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393:2428-38.[PubMed Abstract] -
- 42.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316:171-81.[PubMed Abstract] -
- 43.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820-9.[PubMed Abstract] -
- 44.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384-90.[PubMed Abstract] -
- 45.Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55-64.[PubMed Abstract] -
- 46.Marrazzo J, Tao L, Becker M, et al. HIV Preexposure Prophylaxis With Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women. JAMA. 2024;331:930-7.[PubMed Abstract] -
- 47.Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-92.[PubMed Abstract] -
- 48.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. Sexual assault and abuse and STIs: adolescents and adults. MMWR Recomm Rep. 2021;70(No. RR-4):1-187.
- 49.ACOG Committee Opinion No. 777: Sexual Assault. Obstet Gynecol. 2019;133:e296-e302.[ACOG] -
- 50.Crawford-Jakubiak JE, Alderman EM, Leventhal JM, et al. Care of the Adolescent After an Acute Sexual Assault. Pediatrics. 2017;139:e20164243.[PubMed Abstract] -
- 51.Cherabie JN, Gleason E, Munigala S, et al. Post-exposure prophylaxis for human immunodeficiency virus after sexual assault in a Midwestern U.S. emergency department. Am J Emerg Med. 2021;49:117-23.[PubMed Abstract] -
- 52.Ortega B, Thayer J, Chen L, Steblin S, Mhaskar RS, Straub DM. nPEP protocol implementation and evaluation at a local US Crisis Center. AIDS Care. 2022;34:1268-75.[PubMed Abstract] -
- 53.Roland ME, Myer L, Martin LJ, et al. Preventing human immunodeficiency virus infection among sexual assault survivors in Cape Town, South Africa: an observational study. AIDS Behav. 2012;16:990-8.[PubMed Abstract] -
- 54.Draughon Moret JE, Hauda WE 2nd, Price B, Sheridan DJ. Nonoccupational Postexposure Human Immunodeficiency Virus Prophylaxis: Acceptance Following Sexual Assault. Nurs Res. 2016;65:47-54.[PubMed Abstract] -
- 55.Draughon JE, Sheridan DJ. Nonoccupational postexposure prophylaxis following sexual assault in industrialized low-HIV-prevalence countries: a review. Psychol Health Med. 2012;17:235-54.[PubMed Abstract] -
- 56.Chacko L, Ford N, Sbaiti M, Siddiqui R. Adherence to HIV post-exposure prophylaxis in victims of sexual assault: a systematic review and meta-analysis. Sex Transm Infect. 2012;88:335-41.[PubMed Abstract] -
- 57.Loutfy MR, Macdonald S, Myhr T, et al. Prospective cohort study of HIV post-exposure prophylaxis for sexual assault survivors. Antivir Ther. 2008;13:87-95.[PubMed Abstract] -
- 58.Bogoch II, Scully EP, Zachary KC, et al. Patient attrition between the emergency department and clinic among individuals presenting for HIV nonoccupational postexposure prophylaxis. Clin Infect Dis. 2014;58:1618-24.[PubMed Abstract] -
- 59.Jain S, Krakower DS, Mayer KH. The Transition From Postexposure Prophylaxis to Preexposure Prophylaxis: An Emerging Opportunity for Biobehavioral HIV Prevention. Clin Infect Dis. 2015;60 Suppl 3:S200-4.[PubMed Abstract] -
- 60.Bachmann LH, Barbee LA, Chan P, et al. CDC Clinical Guidelines on the Use of Doxycycline Postexposure Prophylaxis for Bacterial Sexually Transmitted Infection Prevention, United States, 2024. MMWR Recomm Rep. 2024;73:1-8.[PubMed Abstract] -
- 61.Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.[PubMed Abstract] -
- 62.Centers for Disease Control and Prevention (CDC). Hepatitis C Surveillance 2023. Published April 2025.[CDC] -
- 63.Bhattacharya D, Aronsohn A, Price J, Lo Re V. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2023 May 25;ciad319.[PubMed Abstract] -
Additional References
- Al-Hajjar SH, Frayha HH, Al-Hazmi M, et al. Prevention of HIV-1 transmission with postexposure prophylaxis after inadvertent infected blood transfusion. AIDS. 2014;28:1539-41.[PubMed Abstract] -
- Anderson PL, Marzinke MA, Glidden DV. Updating the Adherence-Response for Oral Emtricitabine/Tenofovir Disoproxil Fumarate for Human Immunodeficiency Virus Pre-Exposure Prophylaxis Among Cisgender Women. Clin Infect Dis. 2023;76:1850-3.[PubMed Abstract] -
- Chauveau M, Billaud E, Bonnet B, et al. Tenofovir DF/emtricitabine/rilpivirine as HIV post-exposure prophylaxis: results from a multicentre prospective study. J Antimicrob Chemother. 2019;74:1021-7.[PubMed Abstract] -
- Dobard C, Sharma S, Parikh UM, et al. Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med. 2014;6:227ra35.[PubMed Abstract] -
- Foster R, McAllister J, Read TR, et al. Single-tablet emtricitabine-rilpivirine-tenofovir as HIV postexposure prophylaxis in men who have sex with men. Clin Infect Dis. 2015;61:1336-41.[PubMed Abstract] -
- Martin JN, Roland ME, Neilands TB, et al. Use of postexposure prophylaxis against HIV infection following sexual exposure does not lead to increases in high-risk behavior. AIDS. 2004;18:787-92.[PubMed Abstract] -
- Mayer KH, Jones D, Oldenburg C, et al. Optimal HIV Postexposure Prophylaxis Regimen Completion With Single Tablet Daily Elvitegravir/Cobicistat/Tenofovir Disoproxil Fumarate/Emtricitabine Compared With More Frequent Dosing Regimens. J Acquir Immune Defic Syndr. 2017;75:535-539.[PubMed Abstract] -
- Mayer KH, Mimiaga MJ, Gelman M, Grasso C. Raltegravir, tenofovir DF, and emtricitabine for postexposure prophylaxis to prevent the sexual transmission of HIV: safety, tolerability, and adherence. J Acquir Immune Defic Syndr. 2012;59:354-9.[PubMed Abstract] -
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med. 2014;15:13-22.[PubMed Abstract] -
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. Recommendations for Use of Antiretroviral Drugs During Pregnancy. Lack of Experience with Antiretroviral Drugs During Pregnancy and Prior to Pregnancy (Antiretroviral-Naive). June 12, 2025.[HIV.gov] -
- Pretty IA, Anderson GS, Sweet DJ. Human bites and the risk of human immunodeficiency virus transmission. Am J Forensic Med Pathol. 1999;20:232-9.[PubMed Abstract] -
- Sekar VJ, Lefebvre E, Guzman SS, Felicione E, De Pauw M, Vangeneugden T, Hoetelmans RM. Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir Ther. 2008;13:563-9.[PubMed Abstract] -
- Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904-11.[PubMed Abstract] -
- Terzi R, Niero F, Iemoli E, Capetti A, Coen M, Rizzardini G. Late HIV seroconversion after non-occupational postexposure prophylaxis against HIV with concomitant hepatitis C virus seroconversion. AIDS. 2007;21:262-3.[PubMed Abstract] -
Figures
 Figure 1 (Image Series). Tenofovir PEP following SIV-1 Inoculation of MacaquesAbbreviations: SIV = simian immunodeficiency virus; PEP = postexposure prophylaxis; TFV = tenofovir
Figure 1 (Image Series). Tenofovir PEP following SIV-1 Inoculation of MacaquesAbbreviations: SIV = simian immunodeficiency virus; PEP = postexposure prophylaxis; TFV = tenofovir
In this study, investigators inoculated 24 macaques with simian immunodeficiency virus (SIV) and then instituted various postexposure prophylaxis regimens with tenofovir (PMPA), which is (R)-9-(2-phosphonylmethoxypropyl) adenine.Source: Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265-73. Figure 1B. SIV Transmission Based on Timing of Initiation and Duration of PEPSource: Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265-73.
Figure 1B. SIV Transmission Based on Timing of Initiation and Duration of PEPSource: Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265-73.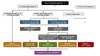 Figure 2. Algorithm for HIV Nonoccupational PEP Use after Possible Sexual Exposure to HIVAbbreviations: PrEP = preexposure prophylaxis; nPEP= nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.
Figure 2. Algorithm for HIV Nonoccupational PEP Use after Possible Sexual Exposure to HIVAbbreviations: PrEP = preexposure prophylaxis; nPEP= nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.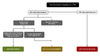 Figure 3. Algorithm for HIV Nonoccupational PEP Use after Possible Injection Drug Use Exposure to HIVAbbreviation: nPEP = nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.
Figure 3. Algorithm for HIV Nonoccupational PEP Use after Possible Injection Drug Use Exposure to HIVAbbreviation: nPEP = nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.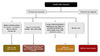 Figure 4. Algorithm for HIV Nonoccupational PEP Use after Other Possible Injection Drug in the Setting of Infective Fluid Splash or Exposure, Needle Injury, orAbbreviation: nPEP= nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.
Figure 4. Algorithm for HIV Nonoccupational PEP Use after Other Possible Injection Drug in the Setting of Infective Fluid Splash or Exposure, Needle Injury, orAbbreviation: nPEP= nonoccupational postexposure prophylaxisSource: Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56.Tables
Table 1. Estimated Per-Act Probability of Acquiring HIV from an Infected Source, by Exposure Act*
Exposure Type Rate for HIV Acquisition per 10,000 Exposures Parenteral Blood transfusion 9,250 Needle sharing during injection drug use 63 Percutaneous (needlestick) 23 Sexual Receptive anal intercourse 138 Insertive anal intercourse 11 Receptive penile-vaginal intercourse 8 Insertive penile-vaginal intercourse 4 Receptive oral intercourse Low Insertive oral intercourse Low Other^ Biting Negligible Spitting Negligible Throwing body fluids (including semen or saliva) Negligible Sharing sex toys Negligible *Factors that may increase the risk of HIV transmission include sexually transmitted infections, acute and late-stage HIV, and high plasma HIV RNA levels. Factors that may decrease the risk include condom use, male circumcision, antiretroviral treatment, and HIV preexposure prophylaxis (PrEP). None of these factors are accounted for in the estimates presented in the table.
^HIV transmission through these exposure routes is technically possible but unlikely and not well documented.Source:- Dominguez KL, Smith DK, Thomas V, et al. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. [CDC]
Table 2. Relative Risks of Factors that Alter Per-Act HIV Transmission Risk for Sexual Exposures*
Cofactor Relative Risk Factors in Source Person with HIV that Increase Transmission Probability High plasma HIV RNA level (log10 copies/mL) 2.89 Genital ulcer disease 2.65 Acute versus asymptomatic stage of disease 7.25 Late versus asymptomatic stage of disease 5.81 Factors that Decrease Transmission Probability Use of antiretroviral medications by persons with HIV Early versus delayed HIV treatment 0.04 Received HIV treatment versus no treatment 0.08 Preexposure prophylaxis taken by person without HIV Among heterosexual couples 0.29 Among men who have sex with men 0.56 Among people who inject drugs 0.52 Condom use 0.20 Male Circumcision (heterosexual partners) Partner without HIV is male 0.50 Partner without HIV is female 0.80 Male circumcision (men who have sex with men) Insertive partner is partner without HIV 0.27 Receptive partner is partner without HIV 1.20 *For a detailed description of data and factors used to generate estimates, see original table in article referenced below. Source:- Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509-19. [PubMed Abstract]
Table 3. 2025 CDC Recommendations for Nonoccupational Postexposure Prophylaxis after Exposure to HIVPreferred and Alternative Regimens for HIV Nonoccupational PEP in Adults and Adolescents*
Adults and Adolescents Aged ≥12 Years (with creatinine clearance ≥50 mL/min and not pregnant) Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine
- Dolutegravir PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
Alternative Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- (Darunavir-cobicistat OR Darunavir and ritonavir) PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
*The regimens within categories are listed in alphabetical order and not to preference. Source:- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Table 4. 2025 CDC Recommendations for Nonoccupational Postexposure Prophylaxis after Exposure to HIVPreferred and Alternative Regimens for HIV Nonoccupational PEP in Pregnant Women*
Pregnant Women (with creatinine clearance ≥50 mL/min) Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine
- Dolutegravir PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
Alternative Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Darunavir and ritonavir (twice daily) PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
*The regimens within categories are listed in alphabetical order and not to preference. Source:- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Table 5. 2025 CDC Recommendations for Nonoccupational Postexposure Prophylaxis after Exposure to HIVPreferred and Alternative Regimens for Nonoccupational HIV PEP for Children up to 12 Years of Age*
Children Aged ≥2 to 12 Years Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine (≥14 kg)¶
- Dolutegravir PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
Alternative Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Darunavir and ritonavir (aged ≥3 years and ≥10 kg) PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)**
- Lopinavir and ritonavir PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)**
Infants and Children Aged ≥4 Weeks to 2 Years Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Dolutegravir (>3 kg) PLUS zidovudine PLUS (emtricitabine OR lamivudine)
Alternative Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Lopinavir and ritonavir PLUS zidovudine PLUS (emtricitabine OR lamivudine)
Neonates Aged ≥14 Days to <4 Weeks Not applicable Health care professionals should consult a local HIV specialist or consult the NCCC PEPline at 844-275-6222.
*The regimens within categories are listed in alphabetical order and not to preference.
¶Bictegravir is available only as part of a fixed-dose combination (FDC) tablet that contains bictegravir-tenofovir alafenamide-emtricitabine; this FDC tablet is recommended as a preferred regimen for children aged ≥2 years and weighing ≥14 kg. Two strengths of bictegravir-tenofovir alafenamide-emtricitabine are available, with dosing according to a child’s weight.
**Tenofovir alafenamide-emtricitabine should not be used with a boosted protease inhibitor if weight <35 kgSource:- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Table 6. 2025 CDC Recommendations for Nonoccupational Postexposure Prophylaxis after Exposure to HIVPreferred and Alternative Regimens for HIV Nonoccupational PEP with Renal Dysfunction or Hepatic Impairment*
Adults and adolescents aged ≥12 years with Moderate Renal Dysfunction (CrCl 30-49 mL/min) Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine
- Dolutegravir PLUS tenofovir alafenamide PLUS (emtricitabine OR lamivudine¶)
Alternative Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Dolutegravir PLUS dose-reduced tenofovir DF**, †† PLUS (emtricitabine OR lamivudine¶)
Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Darunavir-cobicistat-tenofovir alafenamide-emtricitabine
- Darunavir and ritonavir) PLUS (tenofovir alafenamide OR dose-reduced tenofovir DF**, ††) PLUS (emtricitabine OR lamivudine††)
Adults and adolescents aged ≥12 years with Severe Renal Dysfunction (CrCl <30 mL/min) and on hemodialysis) Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine
- Dolutegravir PLUS tenofovir alafenamide PLUS (emtricitabine OR dose-reduced lamivudine††)
Alternative Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Dolutegravir PLUS dose-reduced tenofovir DF** PLUS (emtricitabine OR dose-reduced lamivudine††)
Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Darunavir-cobicistat-tenofovir alafenamide-emtricitabine
- Darunavir and ritonavir PLUS (tenofovir alafenamide OR dose-reduced tenofovir DF**, ††) PLUS (emtricitabine OR dose-reduced lamivudine††)
Adults and adolescents aged ≥12 years with Severe Renal Dysfunction (CrCl <30 mL/min, not on hemodialysis) Not applicable Health care professionals should consult a local HIV specialist or consult the NCCC PEPline at 844-275-6222
Adults and adolescents aged ≥12 years with Hepatic Impairment (Child-Pugh class A or B) Preferred Integrase Strand Transfer Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- Bictegravir-tenofovir alafenamide-emtricitabine
- Dolutegravir PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
Alternative Boosted Protease Inhibitor PLUS Two Nucleoside Reverse Transcriptase Inhibitors
- (Darunavir and cobicistat OR darunavir and ritonavir) PLUS (tenofovir alafenamide OR tenofovir DF) PLUS (emtricitabine OR lamivudine)
Adults and adolescents aged ≥12 years with Hepatic Impairment (Child-Pugh class C) Not applicable Health care professionals should consult a local HIV specialist or consult the NCCC PEPline at 844-275-6222
Abbreviations: CrCl = creatinine clearance
*Regimens within categories are listed in alphabetical order and not to preference.
¶The prescribing information for lamivudine recommends dosage adjustment from 300 mg once daily to 150 mg once daily for patients with CrCl 30–49 mL/min. However, the prescribing information for multiple fixed-dose combination that contain lamivudine recommends no dose adjustment for CrCl 30–49 mL/min. Therefore, no dose adjustment is needed for lamivudine when administered as a standalone tablet or part of a fixed-dose combination tablet.
**Dose-reduced tenofovir DF = 300 mg every 48 hours
††See manufacturer’s package insert for dosing instructions for individual agents or consult the antiretroviral dosing recommendations in adults with renal or hepatic insufficiencySource:- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Table 7. HIV Nonoccupational PEP: Recommended Laboratory Monitoring of Source and Exposed Persons
Test Source Person Exposed to HIV Baseline Baseline 4–6 weeks after exposure 12 weeks after exposure 6 months after exposure All persons evaluated for HIV nPEP Rapid (point-of-care) or laboratory-based HIV Ag/Ab test)† √ √ √§ √ — HIV diagnostic NAT¶ √** √** √§ √ — HBV serology, including: HBsAg, HBsAb, and HBcAb √ √†† — — If HBV nonimmune at baseline HCV antibody testing — √§§ — — If follow-up testing recommended¶¶ HCV RNA NAT √*** — If follow-up testing recommended††† — — Syphilis serology§§§ √ √ √§§§ √§§§ — Gonorrhea NAAT**** √ √ — — — Chlamydia NAAT**** √ √ — — — Pregnancy test†††† — √ √ — — All persons considered for or prescribed nPEP Serum creatinine √ Only if abnormalities at baseline — — Alanine aminotransferase and aspartate aminotransferase √ Only if abnormalities at baseline or symptomatic — — Abbreviations: Abbreviations: Ag/Ab = antigen/antibody combination test; ARV = antiretroviral; HBcAb = hepatitis B core antibody; HBsAb = hepatitis B surface antibody; HBsAg = hepatitis B surface antigen; HBV= hepatitis B virus; HCV = hepatitis C virus; NAT = nucleic acid test; NAAT = nucleic acid amplification test; nPEP = nonoccupational postexposure prophylaxis; PEP = postexposure prophylaxis; STI = sexually transmitted infection.
*Any person diagnosed with an infection or condition through testing should be informed and treated or referred for treatment as needed.
†If a rapid (point-of-care) HIV Ag/Ab test is used, a laboratory-based HIV Ag/Ab test obtained at the same time will increase diagnostic sensitivity. PEP should not be delayed awaiting laboratory results. If the preferred HIV diagnostic test is not accessible, the most sensitive available test should be used.
§HIV testing 4–6 weeks post-nPEP initiation can be deferred for persons who started nPEP within 24 hours of exposure, completed the full PEP course, and are not starting PrEP at this time.
¶NATs that detect HIV RNA include qualitative tests for diagnosis (e.g., HIV-1 RNA assay) and quantitative tests for disease monitoring (e.g., viral load). Diagnostic HIV NATs are recommended because they are more likely than viral load tests to detect very low levels of HIV. If the preferred HIV diagnostic test is not accessible, the most sensitive available test should be used; inability to access HIV NAT should not prevent provision of HIV nPEP to persons with indications.
**HIV NAT recommended at baseline assessment for persons with injectable ARV exposure during the past 6 months.
††HBV PEP recommendations vary by the exposed person’s HBV immune status, and by the source’s HBV status (when information available).
§§Reflex to HCV RNA NAT if HCV antibody test is positive. Add HCV RNA NAT to original order if signs and symptoms of acute HCV infection are present (e.g., hepatic enzyme elevation).
¶¶If follow-up testing is recommended based on the source’s status (e.g., HCV RNA positive or HCV antibody test is positive with unavailable HCV RNA, or if the HCV infection status is unknown), and HCV RNA NAT is negative 3–6 weeks postexposure, a final test for HCV antibodies 4–6 months postexposure is recommended
***HCV RNA NAT is preferred for testing of the source, but if not accessible, HCV antibody testing with reflex HCV RNA NAT if positive is an alternative strategy
†††If follow-up testing is recommended based on the source’s status (e.g., HCV RNA positive or positive HCV antibody with unavailable HCV RNA, or if the HCV infection status is unknown), HCV RNA NAT is recommended for the exposed persons 3–6 weeks postexposure.
§§§STI testing decisions should be made on an individual basis.
¶¶¶If initial syphilis testing negative and infection in the source cannot be ruled out, follow-up testing may be performed 4–6 weeks and 3 months postexposure.
****NAATs are recommended for Chlamydia trachomatis and Neisseria gonorrhoeae at exposure sites (e.g., pharynx, rectum, or vagina) at initial visit and can be repeated 1–2 weeks postexposure if no presumptive treatment was provided and initial test results were negative. Repeat testing can also be done if the person reports symptoms concerning for STIs. Certain experts would also perform a NAAT for Trichomonas vaginalis from a urine or vaginal specimen for persons with vaginas.
††††For all women of child-bearing potential who are not known to be pregnant.Source:- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Table 9. HBV Postexposure Prophylaxis Following Nonoccupational Exposure to HBV*
HBV Status of Person Exposed HBsAg Status of Source HBsAg Positive HBsAg Status Unknown HBsAg Negative Unvaccinated HBIG x 1, and
HBV vaccine series (first dose now)HBV vaccine series (first dose now) HBV vaccine series (first dose now) Partially vaccinated HBIG x 1, and
complete HBV vaccine seriesComplete HBV vaccine series (give next dose in series now) Complete HBV vaccine series (give next dose in series now) Fully vaccinated but response to vaccine unknown HBV vaccine booster dose x 1 (give dose now) HBV vaccine booster dose x 1 (give dose now) No treatment Fully vaccinated with documented response to vaccine† No treatment No treatment No treatment Vaccine nonresponder^ HBIG x 2 (separated by 1 month) HBIG x 2 (separated by 1 month) No treatment Abbreviations: HBV = hepatitis B virus; HBsAg = hepatitis B surface antigen; HBIG = hepatitis B immune globulin
*Exposures include percutaneous (e.g., bite or needlestick) or mucosal exposure to blood or body fluids, sex or needle-sharing contact, or victim of sexual assault/abuse.
†HBV vaccine response is defined as a person with anti-HBs ≥10 mIU/mL after completing a HBV vaccine series.
^HBV vaccine nonresponder is defined as a person with anti-HBs <10 mIU/mL after ≥6 doses of HBV vaccine.Source:- Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31. [PubMed Abstract]
- Tanner MR, O'Shea JG, Byrd KM, et al. Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025. MMWR Recomm Rep. 2025;74:1-56. [PubMed Abstract]
Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
 Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy
Bictegravir-Tenofovir alafenamide-Emtricitabine Biktarvy Darunavir-Cobicistat-Tenofovir alafenamide-Emtricitabine Symtuza
Darunavir-Cobicistat-Tenofovir alafenamide-Emtricitabine Symtuza Dolutegravir-Abacavir-Lamivudine Triumeq
Dolutegravir-Abacavir-Lamivudine Triumeq Dolutegravir-Lamivudine Dovato
Dolutegravir-Lamivudine Dovato Dolutegravir-Rilpivirine Juluca
Dolutegravir-Rilpivirine Juluca Doravirine-Tenofovir DF-Lamivudine Delstrigo
Doravirine-Tenofovir DF-Lamivudine Delstrigo Efavirenz-Tenofovir DF-Emtricitabine Atripla
Efavirenz-Tenofovir DF-Emtricitabine Atripla Elvitegravir-Cobicistat-Tenofovir alafenamide-Emtricitabine Genvoya
Elvitegravir-Cobicistat-Tenofovir alafenamide-Emtricitabine Genvoya Elvitegravir-Cobicistat-Tenofovir DF-Emtricitabine Stribild
Elvitegravir-Cobicistat-Tenofovir DF-Emtricitabine Stribild Rilpivirine-Tenofovir alafenamide-Emtricitabine Odefsey
Rilpivirine-Tenofovir alafenamide-Emtricitabine Odefsey Rilpivirine-Tenofovir DF-Emtricitabine Complera
Rilpivirine-Tenofovir DF-Emtricitabine Complera Fostemsavir Rukobia
Fostemsavir Rukobia Ibalizumab Trogarzo
Ibalizumab Trogarzo Maraviroc Selzentry
Maraviroc Selzentry Dolutegravir Tivicay
Dolutegravir Tivicay Raltegravir Isentress
Raltegravir Isentress Tenofovir alafenamide-Emtricitabine Descovy
Tenofovir alafenamide-Emtricitabine Descovy Tenofovir DF-Emtricitabine Truvada and Multiple Generics
Tenofovir DF-Emtricitabine Truvada and Multiple Generics Doravirine Pifeltro
Doravirine Pifeltro Efavirenz Sustiva
Efavirenz Sustiva Etravirine Intelence
Etravirine Intelence Rilpivirine Edurant
Rilpivirine Edurant



